[English] 日本語
 Yorodumi
Yorodumi- PDB-1wjf: SOLUTION STRUCTURE OF H12C MUTANT OF THE N-TERMINAL ZN BINDING DO... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1wjf | ||||||
|---|---|---|---|---|---|---|---|
| Title | SOLUTION STRUCTURE OF H12C MUTANT OF THE N-TERMINAL ZN BINDING DOMAIN OF HIV-1 INTEGRASE COMPLEXED TO CADMIUM, NMR, 40 STRUCTURES | ||||||
 Components Components | HIV-1 INTEGRASE | ||||||
 Keywords Keywords | ZN-BINDING PROTEIN / AIDS / POLYPROTEIN / HYDROLASE / ASPARTYL PROTEASE | ||||||
| Function / homology |  Function and homology information Function and homology informationHIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding ...HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / membrane Similarity search - Function | ||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||
| Method | SOLUTION NMR / simulated annealing | ||||||
 Authors Authors | Cai, M. / Gronenborn, A.M. / Clore, G.M. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 1998 Journal: Protein Sci. / Year: 1998Title: Solution structure of the His12 --> Cys mutant of the N-terminal zinc binding domain of HIV-1 integrase complexed to cadmium. Authors: Cai, M. / Huang, Y. / Caffrey, M. / Zheng, R. / Craigie, R. / Clore, G.M. / Gronenborn, A.M. #1:  Journal: Nat.Struct.Biol. / Year: 1997 Journal: Nat.Struct.Biol. / Year: 1997Title: Solution Structure of the N-Terminal Zinc Binding Domain of HIV-1 Integrase Authors: Cai, M. / Zheng, R. / Caffrey, M. / Craigie, R. / Clore, G.M. / Gronenborn, A.M. #2:  Journal: Nat.Struct.Biol. / Year: 1997 Journal: Nat.Struct.Biol. / Year: 1997Title: Erratum. Solution Structure of the N-Terminal Zinc Binding Domain of HIV-1 Integrase Authors: Cai, M. / Zheng, R. / Caffrey, M. / Craigie, R. / Clore, G.M. / Gronenborn, A.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1wjf.cif.gz 1wjf.cif.gz | 1.3 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1wjf.ent.gz pdb1wjf.ent.gz | 1.1 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1wjf.json.gz 1wjf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wj/1wjf https://data.pdbj.org/pub/pdb/validation_reports/wj/1wjf ftp://data.pdbj.org/pub/pdb/validation_reports/wj/1wjf ftp://data.pdbj.org/pub/pdb/validation_reports/wj/1wjf | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 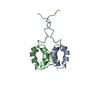
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 6161.949 Da / Num. of mol.: 2 / Mutation: H12C Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Genus: Lentivirus / Cell line: BL21 / Gene: POTENTIAL / Plasmid: PET-15B / Species (production host): Escherichia coli / Gene (production host): T7 / Production host: Human immunodeficiency virus 1 / Genus: Lentivirus / Cell line: BL21 / Gene: POTENTIAL / Plasmid: PET-15B / Species (production host): Escherichia coli / Gene (production host): T7 / Production host:  #2: Chemical | |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|
- Sample preparation
Sample preparation
| Sample conditions | pH: 4.5 / Temperature: 308 K |
|---|---|
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| Software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR software |
| ||||||||||||
| Refinement | Method: simulated annealing / Software ordinal: 1 | ||||||||||||
| NMR ensemble | Conformers calculated total number: 40 / Conformers submitted total number: 40 |
 Movie
Movie Controller
Controller











 PDBj
PDBj


