[English] 日本語
 Yorodumi
Yorodumi- PDB-1utz: Crystal Structure of MMP-12 complexed to (2R)-3-({[4-[(pyridin-4-... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1utz | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of MMP-12 complexed to (2R)-3-({[4-[(pyridin-4-yl)phenyl]-thien-2-yl}carboxamido)(phenyl)propanoic acid | ||||||
 Components Components | MACROPHAGE METALLOELASTASE | ||||||
 Keywords Keywords | HYDROLASE / MACROPHAGE METALLOELASTASE / NON-ZINC CHELATOR / MMP-12 / MMP INHIBITOR / METALLOPROTEASE | ||||||
| Function / homology |  Function and homology information Function and homology informationmacrophage elastase / negative regulation of endothelial cell-matrix adhesion / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway / wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development ...macrophage elastase / negative regulation of endothelial cell-matrix adhesion / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway / wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development / Collagen degradation / response to amyloid-beta / collagen catabolic process / positive regulation of interferon-alpha production / extracellular matrix disassembly / core promoter sequence-specific DNA binding / collagen binding / Degradation of the extracellular matrix / extracellular matrix organization / metalloendopeptidase activity / cellular response to virus / extracellular matrix / protein import into nucleus / endopeptidase activity / sequence-specific DNA binding / serine-type endopeptidase activity / calcium ion binding / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / proteolysis / extracellular space / extracellular region / zinc ion binding / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å MOLECULAR REPLACEMENT / Resolution: 2.5 Å | ||||||
 Authors Authors | Morales, R. / Perrier, S. / Florent, J.M. / Beltra, J. / Dufour, S. / De Mendez, I. / Manceau, P. / Tertre, A. / Moreau, F. / Compere, D. ...Morales, R. / Perrier, S. / Florent, J.M. / Beltra, J. / Dufour, S. / De Mendez, I. / Manceau, P. / Tertre, A. / Moreau, F. / Compere, D. / Dublanchet, A.C. / O'Gara, M. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2004 Journal: J.Mol.Biol. / Year: 2004Title: Crystal Structures of Novel Non-Peptidic, Non-Zinc Chelating Inhibitors Bound to Mmp-12 Authors: Morales, R. / Perrier, S. / Florent, J.M. / Beltra, J. / Dufour, S. / De Mendez, I. / Manceau, P. / Tertre, A. / Moreau, F. / Compere, D. / Dublanchet, A.C. / O'Gara, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1utz.cif.gz 1utz.cif.gz | 83.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1utz.ent.gz pdb1utz.ent.gz | 63.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1utz.json.gz 1utz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ut/1utz https://data.pdbj.org/pub/pdb/validation_reports/ut/1utz ftp://data.pdbj.org/pub/pdb/validation_reports/ut/1utz ftp://data.pdbj.org/pub/pdb/validation_reports/ut/1utz | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1rosC  1uttC  1jk3S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | OUR DYNAMIC LIGHT SCATTERING EXPERIMENTS HAVE SHOWN THATTHE PROTEIN EXISTS AS A MONOMER IN SOLUTION, SUGGESTINGTHAT THE HEXAMER DESCRIBED IN REMARK 350 IS AN ARTIFACTOF CRYSTALLIZATION ONLY. THE PROTEIN IS KNOWN TO FUNCTIONAS A MONOMER |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 17631.648 Da / Num. of mol.: 2 / Fragment: CATALYTIC DOMAIN, RESIDUES 106-264 Source method: isolated from a genetically manipulated source Details: COMPLEXED WITH A SMALL MOLECULE INHIBITOR (2R)-3-({[4-[(PYRIDIN-4-YL)PHENYL]-THIEN-2-YL} CARBOXAMIDO)(PHENYL)PROPANOIC ACID FORMULA C25 H19 N2 O2 S AND ALSO WITH ACETOHYDROXAMIC ACID OR 2- ...Details: COMPLEXED WITH A SMALL MOLECULE INHIBITOR (2R)-3-({[4-[(PYRIDIN-4-YL)PHENYL]-THIEN-2-YL} CARBOXAMIDO)(PHENYL)PROPANOIC ACID FORMULA C25 H19 N2 O2 S AND ALSO WITH ACETOHYDROXAMIC ACID OR 2-HYDROXYAMINO -2-ETHANAL FORMULA, C2H5NO2 Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PGEMEX1 / Production host: HOMO SAPIENS (human) / Plasmid: PGEMEX1 / Production host:  |
|---|
-Non-polymers , 5 types, 144 molecules 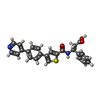
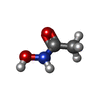







| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-ZN / #5: Chemical | ChemComp-CA / #6: Water | ChemComp-HOH / | |
|---|
-Details
| Compound details | MAY BE INVOLVED IN TISSUE INJURY AND REMODELING |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.37 Å3/Da / Density % sol: 63.54 % |
|---|---|
| Crystal grow | Temperature: 293 K / pH: 8 / Details: 0.1M IMIDAZOLE PH 8.0, 2.0-2.5M NACL, 20 C |
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU MICROMAX-007 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU MICROMAX-007 / Wavelength: 1.5418 |
| Detector | Type: RIGAKU-MSC RAXIS IV++ / Detector: IMAGE PLATE / Date: Jul 15, 2002 / Details: RIGAKU-MSC OSMIC MIRRORS |
| Radiation | Monochromator: NI FILTER / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→99 Å / Num. obs: 26781 / % possible obs: 99.6 % / Observed criterion σ(I): -3 / Redundancy: 6.98 % / Rmerge(I) obs: 0.143 / Net I/σ(I): 13.22 |
| Reflection shell | Resolution: 2.5→2.56 Å / Redundancy: 6.46 % / Rmerge(I) obs: 0.446 / Mean I/σ(I) obs: 4.1 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1JK3 Resolution: 2.5→30 Å / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.5→2.52 Å / Total num. of bins used: 50
|
 Movie
Movie Controller
Controller













 PDBj
PDBj









