+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1tv6 | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIV-1 Reverse Transcriptase Complexed with CP-94,707 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE | ||||||
| Function / homology |  Function and homology information Function and homology informationHIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding ...HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / membrane Similarity search - Function | ||||||
| Biological species |  Human immunodeficiency virus type 1 BH10 Human immunodeficiency virus type 1 BH10 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.8 Å FOURIER SYNTHESIS / Resolution: 2.8 Å | ||||||
 Authors Authors | Pata, J.D. / Stirtan, W.G. / Goldstein, S.W. / Steitz, T.A. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2004 Journal: Proc.Natl.Acad.Sci.USA / Year: 2004Title: Structure of HIV-1 reverse transcriptase bound to an inhibitor active against mutant RTs resistant to other non-nucleoside inhibitors Authors: Pata, J.D. / Stirtan, W.G. / Goldstein, S.W. / Steitz, T.A. #1:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1994 Journal: Proc.Natl.Acad.Sci.USA / Year: 1994Title: Structure of the binding site for nonnucleoside inhibitors of the reverse transcriptase of human immunodeficiency virus type 1 Authors: Smerdon, S.J. / Jaeger, J. / Wang, J. / Kohlstaedt, L.A. / Chirino, A.J. / Friedman, J.M. / Rice, P.A. / Steitz, T.A. #2:  Journal: Science / Year: 1992 Journal: Science / Year: 1992Title: Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor Authors: Kohlstaedt, L.A. / Wang, J. / Friedman, J.M. / Rice, P.A. / Steitz, T.A. | ||||||
| History |
| ||||||
| Remark 600 | HETEROGEN The reference for the bound inhibitor (CP-94,707): Goldstein, S.W., Stirtan, W.G. & ...HETEROGEN The reference for the bound inhibitor (CP-94,707): Goldstein, S.W., Stirtan, W.G. & Sherer, B.A. (2001) US Patent 6,242,461 (Pfizer, Inc.), CAN 135:19641. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1tv6.cif.gz 1tv6.cif.gz | 203.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1tv6.ent.gz pdb1tv6.ent.gz | 162.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1tv6.json.gz 1tv6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tv/1tv6 https://data.pdbj.org/pub/pdb/validation_reports/tv/1tv6 ftp://data.pdbj.org/pub/pdb/validation_reports/tv/1tv6 ftp://data.pdbj.org/pub/pdb/validation_reports/tv/1tv6 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3hvtS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 64517.027 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human immunodeficiency virus type 1 BH10 Human immunodeficiency virus type 1 BH10Genus: Lentivirus / Species: Human immunodeficiency virus 1 / Gene: POL / Plasmid: pKRT / Production host:  |
|---|---|
| #2: Protein | Mass: 51371.035 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human immunodeficiency virus type 1 BH10 Human immunodeficiency virus type 1 BH10Genus: Lentivirus / Species: Human immunodeficiency virus 1 / Gene: POL / Plasmid: pKRT / Production host:  |
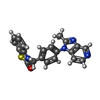
| #3: Chemical | ChemComp-CP9 / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.51 Å3/Da / Density % sol: 65 % |
|---|---|
| Crystal grow | pH: 7 Details: 50 mM bis-Tris-propane, 100 mM ammonium sulfate, 0.2% (w/v) beta-octylglucoside, 10% (v/v) glycerol, 14% (w/v) PEG-8000, pH 7.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1 Å / Beamline: 5.0.2 / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Oct 16, 1999 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→30 Å / Num. all: 37944 / Num. obs: 37716 / % possible obs: 99.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Redundancy: 5.9 % / Rmerge(I) obs: 0.058 / Net I/σ(I): 29.1 |
| Reflection shell | Resolution: 2.8→2.85 Å / Mean I/σ(I) obs: 1.6 / Num. unique all: 1867 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: PDB ENTRY 3HVT Resolution: 2.8→30 Å / Rfactor Rfree error: 0.007 / Data cutoff high absF: 2279111.09 / Data cutoff low absF: 0 / Isotropic thermal model: ANISOTROPIC / Cross valid method: THROUGHOUT / σ(F): 0 Details: This structure was refined against data that were sharpened by applying a B-factor correction of -60 using the CCP4 program CAD. These sharpened data are contained in the structure factor file for this entry.
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 33.312 Å2 / ksol: 0.325981 e/Å3 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 53.6 Å2
| ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.8→30 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Highest resolution: 2.8 Å / Total num. of bins used: 6 /
|
 Movie
Movie Controller
Controller













 PDBj
PDBj