[English] 日本語
 Yorodumi
Yorodumi- PDB-1rnt: RESTRAINED LEAST-SQUARES REFINEMENT OF THE CRYSTAL STRUCTURE OF T... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1rnt | ||||||
|---|---|---|---|---|---|---|---|
| Title | RESTRAINED LEAST-SQUARES REFINEMENT OF THE CRYSTAL STRUCTURE OF THE RIBONUCLEASE T1(ASTERISK)2(PRIME)-GUANYLIC ACID COMPLEX AT 1.9 ANGSTROMS RESOLUTION | ||||||
 Components Components | RIBONUCLEASE T1 ISOZYME | ||||||
 Keywords Keywords | HYDROLASE(ENDORIBONUCLEASE) | ||||||
| Function / homology |  Function and homology information Function and homology informationhyphal tip / ribonuclease T1 / ribonuclease T1 activity / cell septum / RNA endonuclease activity / lyase activity / RNA binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.9 Å X-RAY DIFFRACTION / Resolution: 1.9 Å | ||||||
 Authors Authors | Saenger, W. / Arni, R. / Heinemann, U. / Tokuoka, R. | ||||||
 Citation Citation | Journal: Acta Crystallogr.,Sect.B / Year: 1987 Title: Restrained Least-Squares Refinement of the Crystal Structure of the Ribonuclease T1(Asterisk)2(Prime)-Guanylic Acid Complex at 1.9 Angstroms Resolution Authors: Arni, R. / Heinemann, U. / Maslowska, M. / Tokuoka, R. / Saenger, W. #1:  Journal: J.Biol.Chem. / Year: 1988 Journal: J.Biol.Chem. / Year: 1988Title: Three-Dimensional Structure of the Ribonuclease T1(Asterisk)2(Prime)-Gmp Complex at 1.9-Angstroms Resolution Authors: Arni, R. / Heinemann, U. / Tokuoka, R. / Saenger, W. #2:  Journal: Fresenius Z.Anal.Chem. / Year: 1987 Journal: Fresenius Z.Anal.Chem. / Year: 1987Title: Struktur Und Funktion Des Enzyms Ribonuclease T1 (German) Authors: Arni, R. / Heinemann, U. / Saenger, W. #3:  Journal: Pure Appl.Chem. / Year: 1985 Journal: Pure Appl.Chem. / Year: 1985Title: Mechanism of Guanosine Recognition and RNA Hydrolysis by Ribonuclease T1 Authors: Heinemann, U. / Saenger, W. #4:  Journal: Trends Biochem.Sci.(Pers. Ed.) / Year: 1983 Journal: Trends Biochem.Sci.(Pers. Ed.) / Year: 1983Title: The Structural and Sequence Homology of a Family of Microbial Ribonucleases Authors: Hill, C. / Dodson, G. / Heinemann, U. / Saenger, W. / Mitsui, Y. / Nakamura, K. / Borisov, S. / Tischenko, G. / Polyakov, K. / Pavlovsky, S. #5:  Journal: Jerusalem Symp.Quantum Chem.Biochem. / Year: 1983 Journal: Jerusalem Symp.Quantum Chem.Biochem. / Year: 1983Title: Ribonuclease T1. Mechanism of Specific Guanine Recognition and RNA Hydrolysis Authors: Heinemann, U. / Saenger, W. #6:  Journal: J.Biomol.Struct.Dyn. / Year: 1983 Journal: J.Biomol.Struct.Dyn. / Year: 1983Title: Crystallographic Study of Mechanism of Ribonuclease T1-Catalysed Specific RNA Hydrolysis Authors: Heinemann, U. / Saenger, W. #7:  Journal: Nature / Year: 1982 Journal: Nature / Year: 1982Title: Specific Protein-Nucleic Acid Recognition in Ribonuclease T1-2(Prime)-Guanylic Acid Complex. An X-Ray Study Authors: Heinemann, U. / Saenger, W. #8:  Journal: Eur.J.Biochem. / Year: 1980 Journal: Eur.J.Biochem. / Year: 1980Title: Crystallization of a Complex between Ribonuclease T1 and 2(Prime)-Guanylic Acid Authors: Heinemann, U. / Wernitz, M. / Paehler, A. / Saenger, W. / Menke, G. / Rueterjans, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
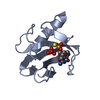
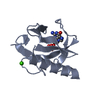
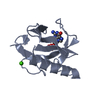
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1rnt.cif.gz 1rnt.cif.gz | 35 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1rnt.ent.gz pdb1rnt.ent.gz | 23.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1rnt.json.gz 1rnt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1rnt_validation.pdf.gz 1rnt_validation.pdf.gz | 838.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1rnt_full_validation.pdf.gz 1rnt_full_validation.pdf.gz | 840.5 KB | Display | |
| Data in XML |  1rnt_validation.xml.gz 1rnt_validation.xml.gz | 8.1 KB | Display | |
| Data in CIF |  1rnt_validation.cif.gz 1rnt_validation.cif.gz | 10.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rn/1rnt https://data.pdbj.org/pub/pdb/validation_reports/rn/1rnt ftp://data.pdbj.org/pub/pdb/validation_reports/rn/1rnt ftp://data.pdbj.org/pub/pdb/validation_reports/rn/1rnt | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: RESIDUES 39 AND 55 ARE CIS-PROLINES. |
- Components
Components
| #1: Protein | Mass: 11094.694 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
|---|---|
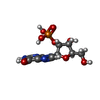
| #2: Chemical | ChemComp-2GP / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.14 Å3/Da / Density % sol: 42.43 % |
|---|---|
| Crystal grow | *PLUS Method: other / Details: Heinemann, U., (1980) Eur. J. Biochem., 109, 109. |
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 1.9 Å / Num. obs: 6788 / % possible obs: 93 % / Observed criterion σ(I): 1 / Num. measured all: 23819 / Rmerge(I) obs: 0.088 |
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.9→6 Å / σ(F): 1 /
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→6 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj