+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1rfa | ||||||
|---|---|---|---|---|---|---|---|
| Title | NMR SOLUTION STRUCTURE OF THE RAS-BINDING DOMAIN OF C-RAF-1 | ||||||
 Components Components | RAF1 | ||||||
 Keywords Keywords | SERINE/THREONINE-PROTEIN KINASE / SIGNAL TRANSDUCTION PROTEIN / SERINE-THREONINE-PROTEIN KINASE complex | ||||||
| Function / homology |  Function and homology information Function and homology informationdeath-inducing signaling complex assembly / intermediate filament cytoskeleton organization / regulation of Rho protein signal transduction / type B pancreatic cell proliferation / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling / Rap1 signalling / insulin secretion involved in cellular response to glucose stimulus / Negative feedback regulation of MAPK pathway / IFNG signaling activates MAPKs ...death-inducing signaling complex assembly / intermediate filament cytoskeleton organization / regulation of Rho protein signal transduction / type B pancreatic cell proliferation / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling / Rap1 signalling / insulin secretion involved in cellular response to glucose stimulus / Negative feedback regulation of MAPK pathway / IFNG signaling activates MAPKs / GP1b-IX-V activation signalling / ERBB2-ERBB3 signaling pathway / neurotrophin TRK receptor signaling pathway / face development / pseudopodium / regulation of cell differentiation / thyroid gland development / extrinsic apoptotic signaling pathway via death domain receptors / somatic stem cell population maintenance / positive regulation of peptidyl-serine phosphorylation / MAP kinase kinase kinase activity / type II interferon-mediated signaling pathway / Schwann cell development / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / negative regulation of protein-containing complex assembly / response to muscle stretch / myelination / CD209 (DC-SIGN) signaling / insulin-like growth factor receptor signaling pathway / thymus development / adenylate cyclase activator activity / wound healing / RAF activation / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / Stimuli-sensing channels / Negative regulation of MAPK pathway / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / Signaling by BRAF and RAF1 fusions / insulin receptor signaling pathway / MAPK cascade / regulation of apoptotic process / mitochondrial outer membrane / protein phosphorylation / protein kinase activity / non-specific serine/threonine protein kinase / positive regulation of MAPK cascade / negative regulation of cell population proliferation / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / negative regulation of apoptotic process / enzyme binding / Golgi apparatus / signal transduction / positive regulation of transcription by RNA polymerase II / mitochondrion / zinc ion binding / ATP binding / identical protein binding / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | SOLUTION NMR | ||||||
 Authors Authors | Emerson, S.D. / Madison, V.S. / Palermo, R.E. / Waugh, D.S. / Scheffler, J.E. / Tsao, K.-L. / Kiefer, S.E. / Liu, S.P. / Fry, D.C. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 1995 Journal: Biochemistry / Year: 1995Title: Solution structure of the Ras-binding domain of c-Raf-1 and identification of its Ras interaction surface. Authors: Emerson, S.D. / Madison, V.S. / Palermo, R.E. / Waugh, D.S. / Scheffler, J.E. / Tsao, K.L. / Kiefer, S.E. / Liu, S.P. / Fry, D.C. #1:  Journal: Biochemistry / Year: 1994 Journal: Biochemistry / Year: 1994Title: Chemical Shift Assignments and Folding Topology of the Ras-Binding Domain of Human Raf-1 as Determined by Heteronuclear Three-Dimensional NMR Spectroscopy Authors: Emerson, S.D. / Waugh, D.S. / Scheffler, J.E. / Tsao, K.L. / Prinzo, K.M. / Fry, D.C. #2:  Journal: J.Biol.Chem. / Year: 1994 Journal: J.Biol.Chem. / Year: 1994Title: Characterization of a 78-Residue Fragment of C-Raf-1 that Comprises a Minimal Binding Domain for the Interaction with Ras-GTP Authors: Scheffler, J.E. / Waugh, D.S. / Bekesi, E. / Kiefer, S.E. / Losardo, J.E. / Neri, A. / Prinzo, K.M. / Tsao, K.L. / Wegrzynski, B. / Emerson, S.D. / al., et | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1rfa.cif.gz 1rfa.cif.gz | 732.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1rfa.ent.gz pdb1rfa.ent.gz | 611.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1rfa.json.gz 1rfa.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rf/1rfa https://data.pdbj.org/pub/pdb/validation_reports/rf/1rfa ftp://data.pdbj.org/pub/pdb/validation_reports/rf/1rfa ftp://data.pdbj.org/pub/pdb/validation_reports/rf/1rfa | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 8975.455 Da / Num. of mol.: 1 Fragment: RAS BINDING DOMAIN, RESIDUES 55 - 132 WITH AN ADDITIONAL ALA AT THE N-TERMINUS Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Organ: FETAL LIVER / Plasmid: PDW333 / Production host: Homo sapiens (human) / Organ: FETAL LIVER / Plasmid: PDW333 / Production host:  References: UniProt: P04049, Transferases; Transferring phosphorus-containing groups; Phosphotransferases with an alcohol group as acceptor |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|
- Sample preparation
Sample preparation
| Crystal grow | *PLUS Method: other |
|---|
- Processing
Processing
| NMR software | Name: CHARMM / Version: 22 / Developer: BROOKS ET AL. / Classification: refinement |
|---|---|
| NMR ensemble | Conformers submitted total number: 30 |
 Movie
Movie Controller
Controller



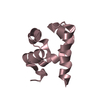









 PDBj
PDBj







