[English] 日本語
 Yorodumi
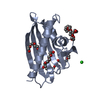
Yorodumi- PDB-1qiw: Calmodulin complexed with N-(3,3,-diphenylpropyl)-N'-[1-R-(3,4-bi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1qiw | ||||||
|---|---|---|---|---|---|---|---|
| Title | Calmodulin complexed with N-(3,3,-diphenylpropyl)-N'-[1-R-(3,4-bis-butoxyphenyl)-ethyl]-propylenediamine (DPD) | ||||||
 Components Components | CALMODULIN | ||||||
 Keywords Keywords | CALCIUM-BINDING PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of store-operated calcium channel activity / : / : / regulation of response to tumor cell / positive regulation of autophagic cell death / DAPK1-calmodulin complex / : / : / : / : ...regulation of store-operated calcium channel activity / : / : / regulation of response to tumor cell / positive regulation of autophagic cell death / DAPK1-calmodulin complex / : / : / : / : / : / : / type 3 metabotropic glutamate receptor binding / establishment of protein localization to membrane / positive regulation of DNA binding / negative regulation of high voltage-gated calcium channel activity / negative regulation of ryanodine-sensitive calcium-release channel activity / organelle localization by membrane tethering / mitochondrion-endoplasmic reticulum membrane tethering / autophagosome membrane docking / negative regulation of calcium ion export across plasma membrane / regulation of cardiac muscle cell action potential / nitric-oxide synthase binding / regulation of synaptic vesicle exocytosis / adenylate cyclase binding / regulation of ryanodine-sensitive calcium-release channel activity / protein phosphatase activator activity / regulation of synaptic vesicle endocytosis / detection of calcium ion / regulation of cardiac muscle contraction / catalytic complex / activation of adenylate cyclase activity / phosphatidylinositol 3-kinase binding / calcium channel inhibitor activity / positive regulation of nitric-oxide synthase activity / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / enzyme regulator activity / titin binding / regulation of calcium-mediated signaling / voltage-gated potassium channel complex / potassium ion transmembrane transport / calcium channel complex / regulation of heart rate / response to amphetamine / adenylate cyclase activator activity / nitric-oxide synthase regulator activity / sarcomere / regulation of cytokinesis / spindle microtubule / calcium channel regulator activity / calcium-mediated signaling / response to calcium ion / G2/M transition of mitotic cell cycle / spindle pole / disordered domain specific binding / calcium-dependent protein binding / myelin sheath / protein autophosphorylation / growth cone / vesicle / transmembrane transporter binding / neuron projection / positive regulation of apoptotic process / protein domain specific binding / calcium ion binding / centrosome / protein kinase binding / protein-containing complex / mitochondrion / nucleoplasm / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Harmat, V. / Bocskei, Z.S. / Vertessy, B.G. / Ovadi, J. / Naray-Szabo, G. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2000 Journal: J.Mol.Biol. / Year: 2000Title: A New Potent Calmodulin Antagonist with Arylalkylamine Structure: Crystallographic, Spectroscopic and Functional Studies Authors: Harmat, V. / Bocskei, Z.S. / Naray-Szabo, G. / Bata, I. / Csutor, A.S. / Hermecz, I. / Aranyi, P. / Szabo, B. / Liliom, K. / Vertessy, B.G. / Ovadi, J. #1: Journal: Proteins: Struct.,Funct., Genet. / Year: 1997 Title: Crystallization and Preliminary Diffraction Analysis of Ca(2+)-Calmodulin-Drug and Apocalmodulin-Drug Complexes. Authors: Vertessy, B.G. / Bocskei, Z.S. / Harmath, V. / Naray-Szabo, G. / Ovadi, J. #2:  Journal: Nat.Struct.Biol. / Year: 1994 Journal: Nat.Struct.Biol. / Year: 1994Title: Trifluoperazine-Induced Conformational Change in Ca (2+)-Calmodulin Authors: Vandonselaar, M. / Hickie, R.A. / Quail, J.W. / Delbaere, L.T.J. #3:  Journal: Biochemistry / Year: 1994 Journal: Biochemistry / Year: 1994Title: Drug Binding by Calmodulin: Crystal Structure of a Calmodulin-Trifluoperazine Complex Authors: Cook, W.J. / Walter, L.J. / Walter, M.R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1qiw.cif.gz 1qiw.cif.gz | 69.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1qiw.ent.gz pdb1qiw.ent.gz | 51.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1qiw.json.gz 1qiw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1qiw_validation.pdf.gz 1qiw_validation.pdf.gz | 1.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1qiw_full_validation.pdf.gz 1qiw_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  1qiw_validation.xml.gz 1qiw_validation.xml.gz | 15.8 KB | Display | |
| Data in CIF |  1qiw_validation.cif.gz 1qiw_validation.cif.gz | 19.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qi/1qiw https://data.pdbj.org/pub/pdb/validation_reports/qi/1qiw ftp://data.pdbj.org/pub/pdb/validation_reports/qi/1qiw ftp://data.pdbj.org/pub/pdb/validation_reports/qi/1qiw | HTTPS FTP |
-Related structure data
| Related structure data |  1qivC  1linS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.638671, 0.193606, -0.744725), Vector: |
- Components
Components
| #1: Protein | Mass: 16721.350 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #2: Chemical | ChemComp-CA / #3: Chemical | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.185 Å3/Da / Density % sol: 44 % Description: THE TWO DOMAINS OF 1LIN WERE USED SEPARATEDLY AS MODELS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, hanging drop / pH: 6 Details: PROTEIN WAS CRYSTALLIZED BY HANGING DROP TECHNIQUE. 50 MM PH=6.0 SODIUM CACODYLATE/HCL BUFFER, 10 MM MGCL2, 10 MM CACL2, 2MM DPD AND 30% PEG 8000 THE CRYSTAL COULD NOT BE REPRODUCED., pH 6.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 7 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 298 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 |
| Detector | Type: RIGAKU IMAGE PLATE / Detector: IMAGE PLATE / Date: Apr 15, 1996 / Details: NORMAL FOCUS |
| Radiation | Monochromator: GRAPHITE(002) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→54.23 Å / Num. obs: 8711 / % possible obs: 69.2 % / Observed criterion σ(I): 3 / Redundancy: 1.7 % / Biso Wilson estimate: 26.7 Å2 / Rmerge(I) obs: 0.137 / Rsym value: 0.093 / Net I/σ(I): 2.1 |
| Reflection shell | Resolution: 2.3→2.42 Å / Redundancy: 1.4 % / Rmerge(I) obs: 0.705 / Mean I/σ(I) obs: 1.3 / Rsym value: 0.513 / % possible all: 25.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1LIN Resolution: 2.3→54.23 Å / Rfactor Rfree error: 0.014 / Data cutoff high absF: 1000000 / Data cutoff low absF: 0.001 / Isotropic thermal model: GROUP / Cross valid method: THROUGHOUT / σ(F): 0 Details: DISORDERED SIDE CHAIN ATOMS OF RESIDUES A2, A7, A47, A78, A79, A86, A114, A123, B6, B13, B69, B77, B82, B84, B123, B127 AND 146 AS WELL AS TRIMETHYL-LYSINES A115, B115 ARE NOT PRESENT IN THE ...Details: DISORDERED SIDE CHAIN ATOMS OF RESIDUES A2, A7, A47, A78, A79, A86, A114, A123, B6, B13, B69, B77, B82, B84, B123, B127 AND 146 AS WELL AS TRIMETHYL-LYSINES A115, B115 ARE NOT PRESENT IN THE STRUCTURE TEMPERATURE FACTORS FOR THE ATOMS IN THE CENTRAL REGIONS (A73-A80, B75- B82),ATOMS AT THE ENDS OF THE POLIPEPTIDE CHAINS (A144-A146, B4-B7, B144) AS WELL AS FOR SOME SURFACE RESIDUES (A45, A84, A107, A112, B107, B119, B126) ARE UNUSUALLY HIGH, INDICATING FLEXIBILITY IN THESE PARTS OF THE STRUCTURE. THE C-TERMINAL RESIDUES FOR BOTH CHAINS A AND B WERE NOT SEEN IN THE DENSITY MAPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 39.8 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→54.23 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Rms dev Biso : 5.019 Å2 / Rms dev position: 0.078 Å / Weight Biso : 10 / Weight position: 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.3→2.4 Å / Rfactor Rfree error: 0.029 / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.28 |
 Movie
Movie Controller
Controller











 PDBj
PDBj





