[English] 日本語
 Yorodumi
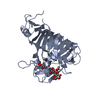
Yorodumi- PDB-1p4o: Structure of Apo unactivated IGF-1R KInase domain at 1.5A resolution. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1p4o | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of Apo unactivated IGF-1R KInase domain at 1.5A resolution. | ||||||
 Components Components | Insulin-like growth factor I receptor protein | ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / IGF-1R / Kinase domain / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationprotein kinase complex / insulin-like growth factor receptor activity / insulin-like growth factor binding / Signaling by Type 1 Insulin-like Growth Factor 1 Receptor (IGF1R) / protein transporter activity / IRS-related events triggered by IGF1R / transcytosis / insulin receptor complex / insulin-like growth factor I binding / positive regulation of protein-containing complex disassembly ...protein kinase complex / insulin-like growth factor receptor activity / insulin-like growth factor binding / Signaling by Type 1 Insulin-like Growth Factor 1 Receptor (IGF1R) / protein transporter activity / IRS-related events triggered by IGF1R / transcytosis / insulin receptor complex / insulin-like growth factor I binding / positive regulation of protein-containing complex disassembly / insulin receptor activity / alphav-beta3 integrin-IGF-1-IGF1R complex / dendritic spine maintenance / regulation of JNK cascade / peptidyl-tyrosine autophosphorylation / insulin binding / amyloid-beta clearance / Respiratory syncytial virus (RSV) attachment and entry / insulin receptor substrate binding / SHC-related events triggered by IGF1R / phosphatidylinositol 3-kinase binding / negative regulation of MAPK cascade / insulin-like growth factor receptor signaling pathway / insulin receptor binding / phosphatidylinositol 3-kinase/protein kinase B signal transduction / cellular response to glucose stimulus / receptor protein-tyrosine kinase / cellular response to amyloid-beta / insulin receptor signaling pathway / positive regulation of cold-induced thermogenesis / protein autophosphorylation / protein tyrosine kinase activity / Extra-nuclear estrogen signaling / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / receptor complex / positive regulation of MAPK cascade / immune response / cilium / positive regulation of cell migration / axon / intracellular membrane-bounded organelle / positive regulation of cell population proliferation / negative regulation of apoptotic process / nucleolus / signal transduction / ATP binding / identical protein binding / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | ||||||
 Authors Authors | Munshi, S. / Kornienko, M. / Hall, D.L. / Darke, P.L. / Waxman, L. / Kuo, L.C. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2003 Journal: Acta Crystallogr.,Sect.D / Year: 2003Title: Structure of apo, unactivated insulin-like growth factor-1 receptor kinase at 1.5 A resolution. Authors: Munshi, S. / Hall, D.L. / Kornienko, M. / Darke, P.L. / Kuo, L.C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1p4o.cif.gz 1p4o.cif.gz | 146.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1p4o.ent.gz pdb1p4o.ent.gz | 115.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1p4o.json.gz 1p4o.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/p4/1p4o https://data.pdbj.org/pub/pdb/validation_reports/p4/1p4o ftp://data.pdbj.org/pub/pdb/validation_reports/p4/1p4o ftp://data.pdbj.org/pub/pdb/validation_reports/p4/1p4o | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 36656.961 Da / Num. of mol.: 2 / Fragment: kinase domain / Mutation: E1067A, E1069A Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: IGF1R / Cell line (production host): SF9 / Production host: Homo sapiens (human) / Gene: IGF1R / Cell line (production host): SF9 / Production host:  #2: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.41 Å3/Da / Density % sol: 48.89 % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: 8% PEG 8k, 0.1M LiCl, 10% ethylene glycol, 10% glycerol and 0.1M Tris-HCl buffer, pH 8.5, VAPOR DIFFUSION, SITTING DROP, temperature 277K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→20 Å / Num. obs: 98666 / % possible obs: 90 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Rmerge(I) obs: 0.079 / Net I/σ(I): 19 |
| Reflection shell | Resolution: 1.5→1.55 Å / Rmerge(I) obs: 0.36 / Mean I/σ(I) obs: 1.7 / % possible all: 53 |
| Reflection | *PLUS Lowest resolution: 20 Å / % possible obs: 90 % / Num. measured all: 1497973 |
| Reflection shell | *PLUS Highest resolution: 1.5 Å / % possible obs: 53 % / Num. unique obs: 5844 / Rmerge(I) obs: 0.368 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.5→20 Å / σ(F): 0 / Stereochemistry target values: Engh & Huber MOLECULAR REPLACEMENT / Resolution: 1.5→20 Å / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→20 Å
| |||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 6 Å / Num. reflection obs: 89907 / % reflection Rfree: 10 % | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj







