[English] 日本語
 Yorodumi
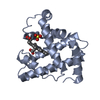
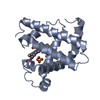
Yorodumi- PDB-1myh: HIGH RESOLUTION X-RAY STRUCTURES OF PIG METMYOGLOBIN AND TWO CD3 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1myh | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIGH RESOLUTION X-RAY STRUCTURES OF PIG METMYOGLOBIN AND TWO CD3 MUTANTS MB(LYS45-> ARG) AND MB(LYS45-> SER) | ||||||
 Components Components | MYOGLOBIN | ||||||
 Keywords Keywords | OXYGEN STORAGE | ||||||
| Function / homology |  Function and homology information Function and homology informationIntracellular oxygen transport / Oxidoreductases; Acting on other nitrogenous compounds as donors / nitrite reductase activity / oxygen transport / sarcoplasm / Oxidoreductases; Acting on a peroxide as acceptor; Peroxidases / removal of superoxide radicals / oxygen carrier activity / peroxidase activity / oxygen binding ...Intracellular oxygen transport / Oxidoreductases; Acting on other nitrogenous compounds as donors / nitrite reductase activity / oxygen transport / sarcoplasm / Oxidoreductases; Acting on a peroxide as acceptor; Peroxidases / removal of superoxide radicals / oxygen carrier activity / peroxidase activity / oxygen binding / heme binding / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.9 Å X-RAY DIFFRACTION / Resolution: 1.9 Å | ||||||
 Authors Authors | Smerdon, S.J. / Oldfield, T.J. / Wilkinson, A.J. / Dauter, Z. / Petratos, K. / Wilson, K.S. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 1992 Journal: Biochemistry / Year: 1992Title: High-resolution X-ray structures of pig metmyoglobin and two CD3 mutants: Mb(Lys45----Arg) and Mb(Lys45----Ser). Authors: Oldfield, T.J. / Smerdon, S.J. / Dauter, Z. / Petratos, K. / Wilson, K.S. / Wilkinson, A.J. #1:  Journal: Acta Crystallogr.,Sect.B / Year: 1990 Journal: Acta Crystallogr.,Sect.B / Year: 1990Title: Determination of the Crystal Structure of Recombinant Pig Myoglobin by Molecular Replacement and its Refinement Authors: Smerdon, S.J. / Oldfield, T.J. / Dodson, E.J. / Dodson, G.G. / Hubbard, R.E. / Wilkinson, A.J. #2:  Journal: Protein Eng. / Year: 1988 Journal: Protein Eng. / Year: 1988Title: Apomyoglobin as a Molecular Recognition Surface: Expression, Reconstitution and Crystallisation of Recombinant Porcine Myoglobin in Escherichia Coli Authors: Dodson, G.G. / Hubbard, R.E. / Oldfield, T.J. / Smerdon, S.J. / Wilkinson, A.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1myh.cif.gz 1myh.cif.gz | 79.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1myh.ent.gz pdb1myh.ent.gz | 58.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1myh.json.gz 1myh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/my/1myh https://data.pdbj.org/pub/pdb/validation_reports/my/1myh ftp://data.pdbj.org/pub/pdb/validation_reports/my/1myh ftp://data.pdbj.org/pub/pdb/validation_reports/my/1myh | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: RESIDUES 152 AND 153 ARE DISORDERED AND NOT DEFINED IN DENSITY MAPS. 2: HOH 48 AND HOH 58 ARE WATER MOLECULES IN THE HEME POCKET LIGATED TO THE HEME IRON AT DISTANCES OF 2.49 AND 2.33 ANGSTROMS, RESPECTIVELY. |
- Components
Components
| #1: Protein | Mass: 17011.461 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  #2: Chemical | #3: Chemical | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.64 Å3/Da / Density % sol: 66.22 % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 15 ℃ / pH: 7.1 / Method: vapor diffusion, hanging dropDetails: taken from Dodson, G. et al(1975). Nature, 257, 758. | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 1.9 Å / Num. obs: 27123 / Num. measured all: 37845 / Rmerge(I) obs: 0.114 |
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.9→10 Å /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: PROLSQ / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.227 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller






















 PDBj
PDBj








