+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 1mw4 | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Solution structure of the human Grb7-SH2 domain in complex with a 10 amino acid peptide pY1139 | ||||||
 要素 要素 |
| ||||||
 キーワード キーワード | HORMONE/GROWTH FACTOR/TRANSFERASE / SH2 domain in complex with a ligand / HORMONE-GROWTH FACTOR-TRANSFERASE COMPLEX | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of immature T cell proliferation in thymus / ERBB3:ERBB2 complex / ERBB2-ERBB4 signaling pathway / GRB7 events in ERBB2 signaling / RNA polymerase I core binding / immature T cell proliferation in thymus / semaphorin receptor complex / regulation of microtubule-based process / ErbB-3 class receptor binding / motor neuron axon guidance ...negative regulation of immature T cell proliferation in thymus / ERBB3:ERBB2 complex / ERBB2-ERBB4 signaling pathway / GRB7 events in ERBB2 signaling / RNA polymerase I core binding / immature T cell proliferation in thymus / semaphorin receptor complex / regulation of microtubule-based process / ErbB-3 class receptor binding / motor neuron axon guidance / Sema4D induced cell migration and growth-cone collapse / RND1 GTPase cycle / PLCG1 events in ERBB2 signaling / ERBB2-EGFR signaling pathway / ERBB2 Activates PTK6 Signaling / neurotransmitter receptor localization to postsynaptic specialization membrane / enzyme-linked receptor protein signaling pathway / neuromuscular junction development / positive regulation of Rho protein signal transduction / ERBB2-ERBB3 signaling pathway / Drug-mediated inhibition of ERBB2 signaling / Resistance of ERBB2 KD mutants to trastuzumab / Resistance of ERBB2 KD mutants to sapitinib / Resistance of ERBB2 KD mutants to tesevatinib / Resistance of ERBB2 KD mutants to neratinib / Resistance of ERBB2 KD mutants to osimertinib / Resistance of ERBB2 KD mutants to afatinib / Resistance of ERBB2 KD mutants to AEE788 / Resistance of ERBB2 KD mutants to lapatinib / Drug resistance in ERBB2 TMD/JMD mutants / positive regulation of MAP kinase activity / positive regulation of transcription by RNA polymerase I / ERBB2 Regulates Cell Motility / semaphorin-plexin signaling pathway / oligodendrocyte differentiation / RET signaling / PI3K events in ERBB2 signaling / positive regulation of protein targeting to membrane / regulation of angiogenesis / regulation of ERK1 and ERK2 cascade / Schwann cell development / coreceptor activity / Tie2 Signaling / Signaling by ERBB2 / TFAP2 (AP-2) family regulates transcription of growth factors and their receptors / myelination / transmembrane receptor protein tyrosine kinase activity / stress granule assembly / phosphatidylinositol binding / GRB2 events in ERBB2 signaling / Downstream signal transduction / positive regulation of cell adhesion / SHC1 events in ERBB2 signaling / Downregulation of ERBB2:ERBB3 signaling / cell surface receptor protein tyrosine kinase signaling pathway / basal plasma membrane / Constitutive Signaling by Overexpressed ERBB2 / cellular response to epidermal growth factor stimulus / peptidyl-tyrosine phosphorylation / positive regulation of translation / positive regulation of epithelial cell proliferation / cell projection / neuromuscular junction / phosphatidylinositol 3-kinase/protein kinase B signal transduction / wound healing / Signaling by ERBB2 TMD/JMD mutants / Signaling by SCF-KIT / receptor protein-tyrosine kinase / Signaling by ERBB2 ECD mutants / receptor tyrosine kinase binding / Signaling by ERBB2 KD Mutants / cellular response to growth factor stimulus / ruffle membrane / Downregulation of ERBB2 signaling / epidermal growth factor receptor signaling pathway / neuron differentiation / cytoplasmic stress granule / Constitutive Signaling by Aberrant PI3K in Cancer / transmembrane signaling receptor activity / PIP3 activates AKT signaling / myelin sheath / heart development / presynaptic membrane / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / RAF/MAP kinase cascade / positive regulation of cell growth / protein tyrosine kinase activity / basolateral plasma membrane / early endosome / cell surface receptor signaling pathway / cell population proliferation / protein phosphorylation / receptor complex / endosome membrane / negative regulation of translation / positive regulation of MAPK cascade / intracellular signal transduction / positive regulation of cell migration / apical plasma membrane / protein heterodimerization activity 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 | 溶液NMR / torsion angle dynamics | ||||||
 データ登録者 データ登録者 | Ivancic, M. / Lyons, B.A. | ||||||
 引用 引用 |  ジャーナル: J.BIOMOL.NMR / 年: 2003 ジャーナル: J.BIOMOL.NMR / 年: 2003タイトル: Solution structure of the human Grb7-SH2 domain/erbB2 peptide complex and structural basis for Grb7 binding to ErbB2 著者: Ivancic, M. / Daly, R.J. / Lyons, B.A. #1:  ジャーナル: J.Biomol.NMR / 年: 2002 ジャーナル: J.Biomol.NMR / 年: 2002タイトル: Assignment of Backbone 1H, 13C, and 15N Resonances of Human Grb7-SH2 Domain in Complex with a Phosphorylated Peptide Ligand 著者: Brescia, P.J. / Ivancic, M. / Lyons, B.A. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  1mw4.cif.gz 1mw4.cif.gz | 414.6 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb1mw4.ent.gz pdb1mw4.ent.gz | 337.7 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  1mw4.json.gz 1mw4.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  1mw4_validation.pdf.gz 1mw4_validation.pdf.gz | 367.7 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  1mw4_full_validation.pdf.gz 1mw4_full_validation.pdf.gz | 549.6 KB | 表示 | |
| XML形式データ |  1mw4_validation.xml.gz 1mw4_validation.xml.gz | 55.5 KB | 表示 | |
| CIF形式データ |  1mw4_validation.cif.gz 1mw4_validation.cif.gz | 69.3 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/mw/1mw4 https://data.pdbj.org/pub/pdb/validation_reports/mw/1mw4 ftp://data.pdbj.org/pub/pdb/validation_reports/mw/1mw4 ftp://data.pdbj.org/pub/pdb/validation_reports/mw/1mw4 | HTTPS FTP |
-関連構造データ
| 類似構造データ | |
|---|---|
| その他のデータベース |
|
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 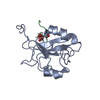
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR アンサンブル |
|
- 要素
要素
| #1: タンパク質 | 分子量: 13690.711 Da / 分子数: 1 / 断片: SH2 domain / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / プラスミド: pGEX2T / 発現宿主: Homo sapiens (ヒト) / プラスミド: pGEX2T / 発現宿主:  |
|---|---|
| #2: タンパク質・ペプチド | 分子量: 1266.205 Da / 分子数: 1 / 断片: SH2 domain binding site / 由来タイプ: 合成 詳細: The peptide was chemically synthesized. The sequence of the peptide is naturally found in Homo sapiens (human). 参照: UniProt: P04626, receptor protein-tyrosine kinase |
| Has protein modification | Y |
-実験情報
-実験
| 実験 | 手法: 溶液NMR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR実験 |
| ||||||||||||||||
| NMR実験の詳細 | Text: The structure was determined using triple-resonance NMR spectroscopy. |
- 試料調製
試料調製
| 詳細 | 内容: 0.7mM Grb7 SH2 U-15N, 13C, 50mM acetate / 溶媒系: 90% H2O/10% D2O |
|---|---|
| 試料状態 | イオン強度: 100 mM NaCl / pH: 6.6 / 圧: 1 atm / 温度: 298 K |
| 結晶化 | *PLUS 手法: other / 詳細: NMR |
-NMR測定
| 放射 | プロトコル: SINGLE WAVELENGTH / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray |
|---|---|
| 放射波長 | 相対比: 1 |
| NMRスペクトロメーター | タイプ: Varian INOVA / 製造業者: Varian / モデル: INOVA / 磁場強度: 500 MHz |
- 解析
解析
| NMR software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 手法: torsion angle dynamics / ソフトェア番号: 1 詳細: THE STRUCTURE IS BASED ON A TOTAL OF 1904 RESTRAINTS: 1660 ARE NOE DERIVED, 120 ARE DIHEDRAL RESTRAINTS AND 124 ARE DISTANCE RESTRAINTS FROM HYDROGEN BONDS. | ||||||||||||||||||||
| 代表構造 | 選択基準: closest to the average | ||||||||||||||||||||
| NMRアンサンブル | コンフォーマー選択の基準: structures with favorable non-bond energy 計算したコンフォーマーの数: 50 / 登録したコンフォーマーの数: 10 |
 ムービー
ムービー コントローラー
コントローラー












 PDBj
PDBj











