[English] 日本語
 Yorodumi
Yorodumi- PDB-1mmb: COMPLEX OF BB94 WITH THE CATALYTIC DOMAIN OF MATRIX METALLOPROTEI... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1mmb | ||||||
|---|---|---|---|---|---|---|---|
| Title | COMPLEX OF BB94 WITH THE CATALYTIC DOMAIN OF MATRIX METALLOPROTEINASE-8 | ||||||
 Components Components | MATRIX METALLOPROTEINASE-8 | ||||||
 Keywords Keywords | HYDROLASE (METALLOPROTEASE) / HYDROLASE / METALLOPROTEASE / METZINCINS / COLLAGEN DEGRADATION | ||||||
| Function / homology |  Function and homology information Function and homology informationneutrophil collagenase / tumor necrosis factor binding / positive regulation of microglial cell activation / positive regulation of tumor necrosis factor-mediated signaling pathway / positive regulation of neuroinflammatory response / Activation of Matrix Metalloproteinases / endodermal cell differentiation / Collagen degradation / collagen catabolic process / extracellular matrix disassembly ...neutrophil collagenase / tumor necrosis factor binding / positive regulation of microglial cell activation / positive regulation of tumor necrosis factor-mediated signaling pathway / positive regulation of neuroinflammatory response / Activation of Matrix Metalloproteinases / endodermal cell differentiation / Collagen degradation / collagen catabolic process / extracellular matrix disassembly / Degradation of the extracellular matrix / extracellular matrix organization / metalloendopeptidase activity / : / specific granule lumen / positive regulation of tumor necrosis factor production / tertiary granule lumen / peptidase activity / cellular response to lipopolysaccharide / endopeptidase activity / serine-type endopeptidase activity / Neutrophil degranulation / proteolysis / extracellular space / extracellular region / zinc ion binding Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.1 Å X-RAY DIFFRACTION / Resolution: 2.1 Å | ||||||
 Authors Authors | Bode, W. / Grams, F. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 1995 Journal: Biochemistry / Year: 1995Title: Structure determination and analysis of human neutrophil collagenase complexed with a hydroxamate inhibitor. Authors: Grams, F. / Crimmin, M. / Hinnes, L. / Huxley, P. / Pieper, M. / Tschesche, H. / Bode, W. #1:  Journal: Eur.J.Biochem. / Year: 1995 Journal: Eur.J.Biochem. / Year: 1995Title: X-Ray Structures of Human Neutrophil Collagenase Complexed with Peptide Hydroxamate and Peptide Thiol Inhibitors. Implications for Substrate Binding and Rational Drug Design Authors: Grams, F. / Reinemer, P. / Powers, J.C. / Kleine, T. / Pieper, M. / Tschesche, H. / Huber, R. / Bode, W. #2:  Journal: FEBS Lett. / Year: 1994 Journal: FEBS Lett. / Year: 1994Title: Structural Implications for the Role of the N Terminus in the 'Superactivation' of Collagenases. A Crystallographic Study Authors: Reinemer, P. / Grams, F. / Huber, R. / Kleine, T. / Schnierer, S. / Piper, M. / Tschesche, H. / Bode, W. #3:  Journal: Embo J. / Year: 1994 Journal: Embo J. / Year: 1994Title: The X-Ray Crystal Structure of the Catalytic Domain of Human Neutrophil Collagenase Inhibited by a Substrate Analogue Reveals the Essentials for Catalysis and Specificity Authors: Bode, W. / Reinemer, P. / Huber, R. / Kleine, T. / Schnierer, S. / Tschesche, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
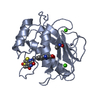
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1mmb.cif.gz 1mmb.cif.gz | 61.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1mmb.ent.gz pdb1mmb.ent.gz | 45 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1mmb.json.gz 1mmb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mm/1mmb https://data.pdbj.org/pub/pdb/validation_reports/mm/1mmb ftp://data.pdbj.org/pub/pdb/validation_reports/mm/1mmb ftp://data.pdbj.org/pub/pdb/validation_reports/mm/1mmb | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 18111.744 Da / Num. of mol.: 1 / Fragment: CATALYTIC DOMAIN Source method: isolated from a genetically manipulated source Details: MMP-8 IS IDENTICAL TO THE HUMAN NEUTROPHIL, COLLAGENASE Source: (gene. exp.)  Homo sapiens (human) / Organ: NEUTROPHILS / Production host: Homo sapiens (human) / Organ: NEUTROPHILS / Production host:  | ||||||
|---|---|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-BAT / | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.37 Å3/Da / Density % sol: 41 % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 22 ℃ / pH: 6 / Method: vapor diffusion, hanging drop | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction source | Wavelength: 1.5418 |
|---|---|
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Num. obs: 9465 / % possible obs: 89.1 % / Rmerge(I) obs: 0.103 |
| Reflection | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 9999 Å / Num. all: 32085 / Num. measured all: 33465 / Rmerge(I) obs: 0.103 |
| Reflection shell | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 2.15 Å / % possible obs: 34.8 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.1→8 Å /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.189 / Rfactor Rwork: 0.189 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 2.17 Å / Rfactor obs: 0.295 |
 Movie
Movie Controller
Controller











 PDBj
PDBj