[English] 日本語
 Yorodumi
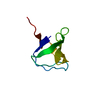
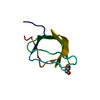
Yorodumi- PDB-1mm3: Solution structure of the 2nd PHD domain from Mi2b with C-termina... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1mm3 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Solution structure of the 2nd PHD domain from Mi2b with C-terminal loop replaced by corresponding loop from WSTF | ||||||
 Components Components | Mi2-beta(Chromodomain helicase-DNA-binding protein 4) and transcription factor WSTF | ||||||
 Keywords Keywords | DNA binding protein/Transcription / PHD / zinc finger / protein scaffold / DNA binding protein-Transcription COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationcerebellar granule cell to Purkinje cell synapse / terminal button organization / NuRD complex / regulation of cell fate specification / NGF-stimulated transcription / regulation of stem cell differentiation / ATP-dependent chromatin remodeler activity / regulation of synapse assembly / RNA Polymerase I Transcription Initiation / site of DNA damage ...cerebellar granule cell to Purkinje cell synapse / terminal button organization / NuRD complex / regulation of cell fate specification / NGF-stimulated transcription / regulation of stem cell differentiation / ATP-dependent chromatin remodeler activity / regulation of synapse assembly / RNA Polymerase I Transcription Initiation / site of DNA damage / Transcriptional regulation of brown and beige adipocyte differentiation by EBF2 / Regulation of TP53 Activity through Acetylation / Regulation of PTEN gene transcription / transcription coregulator binding / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / Regulation of endogenous retroelements by KRAB-ZFP proteins / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / helicase activity / double-strand break repair via homologous recombination / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / RNA polymerase II transcription regulator complex / histone deacetylase binding / transcription corepressor activity / histone binding / Potential therapeutics for SARS / RNA polymerase II-specific DNA-binding transcription factor binding / chromosome, telomeric region / chromatin remodeling / negative regulation of gene expression / negative regulation of DNA-templated transcription / chromatin binding / centrosome / positive regulation of DNA-templated transcription / chromatin / negative regulation of transcription by RNA polymerase II / ATP hydrolysis activity / protein-containing complex / DNA binding / zinc ion binding / nucleoplasm / ATP binding / membrane / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | SOLUTION NMR / simulated annealing, molecular dynamics, torsion angle dynamics | ||||||
 Authors Authors | Kwan, A.H.Y. / Gell, D.A. / Verger, A. / Crossley, M. / Matthews, J.M. / Mackay, J.P. | ||||||
 Citation Citation |  Journal: structure / Year: 2003 Journal: structure / Year: 2003Title: Engineering a Protein Scaffold from a PHD Finger Authors: Kwan, A.H.Y. / Gell, D.A. / Verger, A. / Crossley, M. / Matthews, J.M. / Mackay, J.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1mm3.cif.gz 1mm3.cif.gz | 356.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1mm3.ent.gz pdb1mm3.ent.gz | 294.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1mm3.json.gz 1mm3.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mm/1mm3 https://data.pdbj.org/pub/pdb/validation_reports/mm/1mm3 ftp://data.pdbj.org/pub/pdb/validation_reports/mm/1mm3 ftp://data.pdbj.org/pub/pdb/validation_reports/mm/1mm3 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 6774.832 Da / Num. of mol.: 1 / Fragment: residues 1-61 Source method: isolated from a genetically manipulated source Details: the C-terminal loop (residue 37-49) was replaced by corresponding loop from WSTF. Source: (gene. exp.)  Homo sapiens (human) / Gene: CHD4 / Plasmid: pGEX-6P / Species (production host): Escherichia coli / Production host: Homo sapiens (human) / Gene: CHD4 / Plasmid: pGEX-6P / Species (production host): Escherichia coli / Production host:  |
|---|---|
| #2: Chemical |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||
| NMR details | Text: This structure was determined mostly using standard 2D homonuclear techniques |
- Sample preparation
Sample preparation
| Details | Contents: 1mM Mi2-phd2-L2wstf protein, 1mM DTT, 10mM sodium phosphate buffer (pH 7.5), 150mM NaCl, 95% H2O, 5% D2O Solvent system: 95% H2O/5% D2O |
|---|---|
| Sample conditions | Ionic strength: 150mM NaCl / pH: 7.5 / Pressure: ambient / Temperature: 298 K |
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| NMR spectrometer | Type: Bruker DRX / Manufacturer: Bruker / Model: DRX / Field strength: 600 MHz |
- Processing
Processing
| NMR software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: simulated annealing, molecular dynamics, torsion angle dynamics Software ordinal: 1 Details: Structure calculations were performed using the package ARIA1.1 (Ambiguous Restraints in Iterative Assignment). Final structures are based on 968 unambiguous NOE-derived distance ...Details: Structure calculations were performed using the package ARIA1.1 (Ambiguous Restraints in Iterative Assignment). Final structures are based on 968 unambiguous NOE-derived distance constraints, 90 sets of ambiguous NOE-derived distance constraints and 37 additional dihedral angle restraints. | ||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 500 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller












 PDBj
PDBj











