[English] 日本語
 Yorodumi
Yorodumi- PDB-1lmc: THE CRYSTAL STRUCTURE OF A COMPLEX BETWEEN BULGECIN, A BACTERIAL ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1lmc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | THE CRYSTAL STRUCTURE OF A COMPLEX BETWEEN BULGECIN, A BACTERIAL METABOLITE, AND LYSOZYME FROM THE RAINBOW TROUT | |||||||||
 Components Components | LYSOZYME | |||||||||
 Keywords Keywords | HYDROLASE (O-GLYCOSYL) | |||||||||
| Function / homology |  Function and homology information Function and homology informationlysozyme / lysozyme activity / defense response to Gram-negative bacterium / killing of cells of another organism / defense response to Gram-positive bacterium / extracellular space Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2 Å X-RAY DIFFRACTION / Resolution: 2 Å | |||||||||
 Authors Authors | Karlsen, S. / Hough, E. | |||||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 1996 Journal: Acta Crystallogr.,Sect.D / Year: 1996Title: Structure of a complex between bulgecin, a bacterial metabolite, and lysozyme from the rainbow trout. Authors: Karlsen, S. / Hough, E. #1:  Journal: To be Published Journal: To be PublishedTitle: The Crystal Structures of Three Complexes between Chitooligosaccharides and Lysozyme from the Rainbow Trout Authors: Karlsen, S. / Hough, E. #2:  Journal: To be Published Journal: To be PublishedTitle: The Refined Crystal Structure of Lysozyme from Rainbow Trout (Oncorhynchus Mykiss) Authors: Karlsen, S. / Eliassen, B.E. / Hansen, L.K. / Larsen, R.L. / Riise, B.W. / Smalaas, A.O. / Hough, E. / Grinde, B. #3:  Journal: Tetrahedron / Year: 1984 Journal: Tetrahedron / Year: 1984Title: Structures of Bulgecins, Bacterial Metabolites with Bulge-Inducing Activity Authors: Shinagawa, S. / Kasahara, F. / Wada, Y. / Harada, S. / Asai, M. | |||||||||
| History |
|
- Structure visualization
Structure visualization
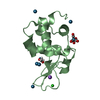
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1lmc.cif.gz 1lmc.cif.gz | 42.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1lmc.ent.gz pdb1lmc.ent.gz | 28.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1lmc.json.gz 1lmc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1lmc_validation.pdf.gz 1lmc_validation.pdf.gz | 801.5 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1lmc_full_validation.pdf.gz 1lmc_full_validation.pdf.gz | 808.2 KB | Display | |
| Data in XML |  1lmc_validation.xml.gz 1lmc_validation.xml.gz | 10.2 KB | Display | |
| Data in CIF |  1lmc_validation.cif.gz 1lmc_validation.cif.gz | 13.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lm/1lmc https://data.pdbj.org/pub/pdb/validation_reports/lm/1lmc ftp://data.pdbj.org/pub/pdb/validation_reports/lm/1lmc ftp://data.pdbj.org/pub/pdb/validation_reports/lm/1lmc | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 14303.068 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: Chemical | ChemComp-BUL / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.22 Å3/Da / Density % sol: 61.78 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 277 K / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction source | Wavelength: 1.5418 Å |
|---|---|
| Detector | Type: ENRAF-NONIUS FAST / Detector: DIFFRACTOMETER / Date: Mar 17, 1994 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Num. obs: 10895 / % possible obs: 86.1 % / Observed criterion σ(I): 3 / Rmerge(I) obs: 0.059 |
| Reflection | *PLUS Highest resolution: 2 Å / Lowest resolution: 14.68 Å / Rmerge(I) obs: 0.059 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2→8 Å / σ(F): 3 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj