+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1lb5 | ||||||
|---|---|---|---|---|---|---|---|
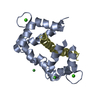
| Title | TRAF6-RANK Complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | SIGNALING PROTEIN / TRAF6-RANK complex | ||||||
| Function / homology |  Function and homology information Function and homology informationmultinuclear osteoclast differentiation / positive regulation of fever generation by positive regulation of prostaglandin secretion / circadian temperature homeostasis / cellular response to zinc ion starvation / tumor necrosis factor receptor activity / interleukin-17A-mediated signaling pathway / protein kinase B binding / TNF receptor superfamily (TNFSF) members mediating non-canonical NF-kB pathway / CD40 signaling pathway / MyD88 dependent cascade initiated on endosome ...multinuclear osteoclast differentiation / positive regulation of fever generation by positive regulation of prostaglandin secretion / circadian temperature homeostasis / cellular response to zinc ion starvation / tumor necrosis factor receptor activity / interleukin-17A-mediated signaling pathway / protein kinase B binding / TNF receptor superfamily (TNFSF) members mediating non-canonical NF-kB pathway / CD40 signaling pathway / MyD88 dependent cascade initiated on endosome / TRAF6 mediated induction of NFkB and MAP kinases upon TLR7/8 or 9 activation / MyD88 cascade initiated on plasma membrane / activation of protein kinase activity / interleukin-17-mediated signaling pathway / CD40 receptor complex / interleukin-33-mediated signaling pathway / protein branched polyubiquitination / toll-like receptor 3 signaling pathway / positive regulation of JUN kinase activity / Regulated proteolysis of p75NTR / TRIF-dependent toll-like receptor signaling pathway / myeloid dendritic cell differentiation / positive regulation of lipopolysaccharide-mediated signaling pathway / positive regulation of osteoclast differentiation / tumor necrosis factor receptor binding / positive regulation of bone resorption / regulation of immunoglobulin production / regulation of canonical NF-kappaB signal transduction / positive regulation of leukocyte adhesion to vascular endothelial cell / ubiquitin conjugating enzyme binding / interleukin-1-mediated signaling pathway / extrinsic component of cytoplasmic side of plasma membrane / TRAF6 mediated IRF7 activation / MyD88:MAL(TIRAP) cascade initiated on plasma membrane / T-helper 1 type immune response / MyD88-dependent toll-like receptor signaling pathway / toll-like receptor 4 signaling pathway / ubiquitin-ubiquitin ligase activity / non-canonical NF-kappaB signal transduction / cytoplasmic pattern recognition receptor signaling pathway / cytokine binding / Fc-epsilon receptor signaling pathway / stimulatory C-type lectin receptor signaling pathway / cellular response to cytokine stimulus / odontogenesis of dentin-containing tooth / regulation of neurotransmitter receptor localization to postsynaptic specialization membrane / response to tumor necrosis factor / TRAF6 mediated NF-kB activation / monocyte chemotaxis / mammary gland alveolus development / protein K63-linked ubiquitination / autophagosome assembly / canonical NF-kappaB signal transduction / lymph node development / bone resorption / response to mechanical stimulus / protein autoubiquitination / positive regulation of type I interferon production / lipid droplet / positive regulation of interleukin-12 production / signaling adaptor activity / IRAK2 mediated activation of TAK1 complex / Alpha-protein kinase 1 signaling pathway / IRAK1 recruits IKK complex / IRAK1 recruits IKK complex upon TLR7/8 or 9 stimulation / antiviral innate immune response / IRAK2 mediated activation of TAK1 complex upon TLR7/8 or 9 stimulation / TICAM1,TRAF6-dependent induction of TAK1 complex / positive regulation of interleukin-2 production / positive regulation of T cell proliferation / response to interleukin-1 / p75NTR recruits signalling complexes / osteoclast differentiation / TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling / lipopolysaccharide-mediated signaling pathway / NF-kB is activated and signals survival / TRAF6-mediated induction of TAK1 complex within TLR4 complex / ossification / NRIF signals cell death from the nucleus / positive regulation of protein ubiquitination / TICAM1, RIP1-mediated IKK complex recruitment / tumor necrosis factor-mediated signaling pathway / JNK (c-Jun kinases) phosphorylation and activation mediated by activated human TAK1 / IKK complex recruitment mediated by RIP1 / Regulation of NF-kappa B signaling / activated TAK1 mediates p38 MAPK activation / TNFR2 non-canonical NF-kB pathway / neural tube closure / positive regulation of non-canonical NF-kappaB signal transduction / : / positive regulation of T cell cytokine production / NOD1/2 Signaling Pathway / TAK1-dependent IKK and NF-kappa-B activation / RING-type E3 ubiquitin transferase / positive regulation of JNK cascade / response to insulin / positive regulation of interleukin-6 production / CLEC7A (Dectin-1) signaling / cytoplasmic side of plasma membrane / FCERI mediated NF-kB activation Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | ||||||
 Authors Authors | Ye, H. / Arron, J.R. / Lamothe, B. / Cirilli, M. / Kobayashi, T. / Shevde, N.K. / Segal, D. / Dzivenu, O. / Vologodskaia, M. / Yim, M. ...Ye, H. / Arron, J.R. / Lamothe, B. / Cirilli, M. / Kobayashi, T. / Shevde, N.K. / Segal, D. / Dzivenu, O. / Vologodskaia, M. / Yim, M. / Du, K. / Singh, S. / Pike, J.W. / Darnay, B.G. / Choi, Y. / Wu, H. | ||||||
 Citation Citation |  Journal: Nature / Year: 2002 Journal: Nature / Year: 2002Title: Distinct molecular mechanism for initiating TRAF6 signalling. Authors: Ye, H. / Arron, J.R. / Lamothe, B. / Cirilli, M. / Kobayashi, T. / Shevde, N.K. / Segal, D. / Dzivenu, O.K. / Vologodskaia, M. / Yim, M. / Du, K. / Singh, S. / Pike, J.W. / Darnay, B.G. / Choi, Y. / Wu, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1lb5.cif.gz 1lb5.cif.gz | 46.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1lb5.ent.gz pdb1lb5.ent.gz | 32.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1lb5.json.gz 1lb5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lb/1lb5 https://data.pdbj.org/pub/pdb/validation_reports/lb/1lb5 ftp://data.pdbj.org/pub/pdb/validation_reports/lb/1lb5 ftp://data.pdbj.org/pub/pdb/validation_reports/lb/1lb5 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 18676.549 Da / Num. of mol.: 1 / Fragment: residues 347-504 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TRAF6 / Production host: Homo sapiens (human) / Gene: TRAF6 / Production host:  |
|---|---|
| #2: Protein/peptide | Mass: 1012.048 Da / Num. of mol.: 1 / Fragment: residues 342-349 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: RANK / Production host: Homo sapiens (human) / Gene: RANK / Production host:  |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.31 Å3/Da / Density % sol: 46.77 % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: PEG8000, Tris, pH 7.5, VAPOR DIFFUSION, HANGING DROP, temperature 293K | ||||||||||||||||||
| Crystal grow | *PLUS Method: unknown | ||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X4A / Beamline: X4A |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Sep 21, 1999 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Relative weight: 1 |
| Reflection | Resolution: 2.4→40 Å / Num. obs: 12396 / % possible obs: 96 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 1 / Biso Wilson estimate: 14.1 Å2 / Rsym value: 0.055 |
| Reflection shell | Resolution: 2.4→2.55 Å / % possible all: 95.8 |
| Reflection | *PLUS Highest resolution: 2 Å / Lowest resolution: 40 Å / Rmerge(I) obs: 0.055 |
| Reflection shell | *PLUS % possible obs: 86.9 % / Rmerge(I) obs: 0.139 |
- Processing
Processing
| Software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: TRAF6 apo form Resolution: 2.4→19.63 Å / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||
| Displacement parameters | Biso mean: 22 Å2 | ||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.4→19.63 Å
| ||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2 Å / Lowest resolution: 20 Å / σ(F): 2 / Rfactor Rfree: 0.242 / Rfactor Rwork: 0.213 | ||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller














 PDBj
PDBj














