+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1k48 | ||||||
|---|---|---|---|---|---|---|---|
| Title | REFINED STRUCTURE AND DISULFIDE PAIRING OF THE KALATA B1 PEPTIDE | ||||||
 Components Components | Kalata B1 | ||||||
 Keywords Keywords | PLANT PROTEIN / CYCLIC PEPTIDE / CYCLOTIDE / DISULFIDE PAIRING / UTEROTONIC | ||||||
| Function / homology | Cyclotide, moebius, conserved site / Cyclotides Moebius subfamily signature. / Cyclotides profile. / Cyclotide / Cyclotide superfamily / Cyclotide family / killing of cells of another organism / defense response to bacterium / Kalata-B1 Function and homology information Function and homology information | ||||||
| Biological species |  Oldenlandia affinis (plant) Oldenlandia affinis (plant) | ||||||
| Method | SOLUTION NMR / torsion angle dynamics | ||||||
| Model type details | minimized average | ||||||
 Authors Authors | Skjeldal, L. / Gran, L. / Sletten, K. / Volkman, B.F. | ||||||
 Citation Citation |  Journal: Arch.Biochem.Biophys. / Year: 2002 Journal: Arch.Biochem.Biophys. / Year: 2002Title: Refined structure and metal binding site of the kalata B1 peptide. Authors: Skjeldal, L. / Gran, L. / Sletten, K. / Volkman, B.F. #1:  Journal: Biochemistry / Year: 1995 Journal: Biochemistry / Year: 1995Title: Elucidation of the Primary and Three-Dimensional Structure of the Uterotonic Polypeptide Kalata B1 | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1k48.cif.gz 1k48.cif.gz | 16.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1k48.ent.gz pdb1k48.ent.gz | 9.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1k48.json.gz 1k48.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1k48_validation.pdf.gz 1k48_validation.pdf.gz | 239.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1k48_full_validation.pdf.gz 1k48_full_validation.pdf.gz | 238.8 KB | Display | |
| Data in XML |  1k48_validation.xml.gz 1k48_validation.xml.gz | 2.2 KB | Display | |
| Data in CIF |  1k48_validation.cif.gz 1k48_validation.cif.gz | 2.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/k4/1k48 https://data.pdbj.org/pub/pdb/validation_reports/k4/1k48 ftp://data.pdbj.org/pub/pdb/validation_reports/k4/1k48 ftp://data.pdbj.org/pub/pdb/validation_reports/k4/1k48 | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 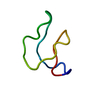
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 2917.345 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: Extracted from African plant kalata-kalata / Source: (natural)  Oldenlandia affinis (plant) / Strain: DC / References: UniProt: P56254 Oldenlandia affinis (plant) / Strain: DC / References: UniProt: P56254 |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|---|
| NMR experiment | Type: 2D NOESY |
| NMR details | Text: STRUCTURE WAS DETERMINED USING NOES FROM A SINGLE 100 MS MIXING TIME NOESY EXPERIMENT |
- Sample preparation
Sample preparation
| Details | Contents: 5 MM KALATA B1, 10% D2O |
|---|---|
| Sample conditions | Ionic strength: 0 / pH: 3 / Pressure: AMBIENT / Temperature: 298.00 K |
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| NMR spectrometer | Type: Bruker DMX / Manufacturer: Bruker / Model: DMX / Field strength: 750 MHz |
- Processing
Processing
| NMR software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: torsion angle dynamics / Software ordinal: 1 Details: STRUCTURES ARE BASED ON A TOTAL OF 333 DISTANCE CONSTRAINTS. THIS INCLUDES 6 UPPER AND 6 LOWER LIMITS DEFINING 3 DISULFIDE BONDS, AS WELL AS 3 UPPER AND 3 LOWER LIMITS DEFINING A PEPTIDE ...Details: STRUCTURES ARE BASED ON A TOTAL OF 333 DISTANCE CONSTRAINTS. THIS INCLUDES 6 UPPER AND 6 LOWER LIMITS DEFINING 3 DISULFIDE BONDS, AS WELL AS 3 UPPER AND 3 LOWER LIMITS DEFINING A PEPTIDE BOND CYCLIZING THE PEPTIDE BACKBONE. RESIDUE NUMBERING FOLLOWS THE ORIGINAL DESCRIPTION OF CITATION 1, EXCEPT THAT FOR THE PURPOSES OF STRUCTURE CALCULATIONS, THE N-TERMINAL RESIDUE WAS TAKEN AS ASN8. THEREFORE, RESIDUES 30-36 IN THIS DEPOSITION CORRESPOND TO RESIDUES 1-7 IN CITATION 1 AND RELATED PDB ENTRY 1KAL. STRUCTURES WERE REFINED IN THE ABSENCE OF ANY ARTIFICIAL CONSTRAINTS DEFINING DISULFIDE BONDS UNTIL ALL NOES HAD BEEN ASSIGNED AND LOW TARGET FUNCTIONS WERE ACHIEVED (TF=0.6). 15 ADDITIONAL CALCULATIONS WERE PERFORMED WITH THESE INPUT DATA AND THE INCLUSION OF CONSTRAINTS DEFINING ALL POSSIBLE DISULFIDE PAIRING COMBINATIONS. THE STRUCTURES CONTAINING DISULFIDES BETWEEN [5(34)-13], [17-29] AND [22-27] DISPLAYED THE LOWEST TARGET FUNCTION (0.74, SECOND LOWEST WAS 1.54). ON THE BASIS OF THIS RESULT AND ANALYSIS OF NOES OBSERVED BETWEEN CYS SIDECHAIN PROTONS, THIS DISULFIDE BONDING ARRANGEMENT WAS ASSUMED TO BE CORRECT AND SERVED AS THE BASIS FOR THIS DEPOSITION. | ||||||||||||||||
| NMR representative | Selection criteria: minimized average structure | ||||||||||||||||
| NMR ensemble | Conformers submitted total number: 1 |
 Movie
Movie Controller
Controller











 PDBj
PDBj