[English] 日本語
 Yorodumi
Yorodumi- PDB-1jcs: CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WI... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1jcs | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH THE PEPTIDE SUBSTRATE TKCVFM AND AN ANALOG OF FARNESYL DIPHOSPHATE | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR / FTASE / PFT / PFTASE / FT / FPT / FARNESYLTRANSFERASE / FARNESYL TRANSFERASE / FARNESYL PROTEIN TRANSFERASE / CAAX / RAS / CANCER / INHIBITOR / TRANSFERASE / transferase-transferase inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationApoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / peptide pheromone maturation / protein farnesylation / protein geranylgeranyltransferase type I / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase ...Apoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / peptide pheromone maturation / protein farnesylation / protein geranylgeranyltransferase type I / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase / protein farnesyltransferase activity / protein farnesyltransferase complex / Rab geranylgeranyltransferase activity / protein geranylgeranylation / regulation of fibroblast proliferation / regulation of microtubule-based movement / geranylgeranyl diphosphate synthase activity / positive regulation of skeletal muscle acetylcholine-gated channel clustering / acetyltransferase activator activity / microtubule associated complex / enzyme-linked receptor protein signaling pathway / positive regulation of Rac protein signal transduction / alpha-tubulin binding / positive regulation of cell cycle / wound healing / receptor tyrosine kinase binding / lipid metabolic process / positive regulation of fibroblast proliferation / fibroblast proliferation / microtubule binding / molecular adaptor activity / cell population proliferation / negative regulation of cell population proliferation / positive regulation of cell population proliferation / negative regulation of apoptotic process / enzyme binding / zinc ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Long, S.B. / Casey, P.J. / Beese, L.S. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2001 Journal: Proc.Natl.Acad.Sci.USA / Year: 2001Title: The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics. Authors: Long, S.B. / Hancock, P.J. / Kral, A.M. / Hellinga, H.W. / Beese, L.S. #1:  Journal: Structure / Year: 2000 Journal: Structure / Year: 2000Title: The Basis for K-Ras4B Binding Specificity to Protein Farnesyltransferase Revealed by 2A Resolution Ternary Complex Structures Authors: Long, S.B. / Casey, P.J. / Beese, L.S. #2:  Journal: Science / Year: 1997 Journal: Science / Year: 1997Title: Crystal Structure of Protein Farnesyltransferase at 2.25A Resolution Authors: Park, H.-W. / Boduluri, S.R. / Moomaw, J.F. / Casey, P.J. / Beese, L.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1jcs.cif.gz 1jcs.cif.gz | 174 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1jcs.ent.gz pdb1jcs.ent.gz | 134.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1jcs.json.gz 1jcs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jc/1jcs https://data.pdbj.org/pub/pdb/validation_reports/jc/1jcs ftp://data.pdbj.org/pub/pdb/validation_reports/jc/1jcs ftp://data.pdbj.org/pub/pdb/validation_reports/jc/1jcs | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1jcqC  1jcrC  1d8dS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
| ||||||||||
| Details | The biological unit of protein farnesyltransferase (FTase) is a heterodimer of alpha and beta subunits. There is one FTase heterodimer per asymmetric unit. |
- Components
Components
-PROTEIN FARNESYLTRANSFERASE, ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 44098.145 Da / Num. of mol.: 1 / Fragment: ALPHA SUBUNIT Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: Q04631, Transferases; Transferring alkyl or aryl groups, other than methyl groups |
|---|---|
| #2: Protein | Mass: 48722.281 Da / Num. of mol.: 1 / Fragment: BETA SUBUNIT Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: Q02293, Transferases; Transferring alkyl or aryl groups, other than methyl groups |
-Protein/peptide , 1 types, 1 molecules C
| #3: Protein/peptide | Mass: 728.942 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: NON-SUBSTRATE INHIBITOR |
|---|
-Non-polymers , 4 types, 440 molecules 
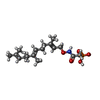





| #4: Chemical | ChemComp-ZN / | ||
|---|---|---|---|
| #5: Chemical | ChemComp-FII / [( | ||
| #6: Chemical | | #7: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.13 Å3/Da / Density % sol: 60.67 % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 5.7 Details: PEG 8000, Ammonium Acetate, DTT, Tris-HCl, pH 5.7, VAPOR DIFFUSION, HANGING DROP at 290K | ||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 17 ℃ | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: May 17, 1999 / Details: Mirrors |
| Radiation | Monochromator: Ni Filter / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→50 Å / Num. all: 58693 / Num. obs: 55202 / % possible obs: 93.8 % / Observed criterion σ(I): 0 / Redundancy: 5.1 % / Biso Wilson estimate: 31.8 Å2 / Rmerge(I) obs: 0.067 / Net I/σ(I): 17.2 |
| Reflection shell | Resolution: 2.2→2.23 Å / Redundancy: 4.98 % / Rmerge(I) obs: 0.269 / Mean I/σ(I) obs: 2.64 / Num. unique all: 1953 / % possible all: 100 |
| Reflection | *PLUS Lowest resolution: 50 Å / Num. obs: 58693 / % possible obs: 99.7 % / Num. measured all: 207941 / Rmerge(I) obs: 0.088 |
| Reflection shell | *PLUS Highest resolution: 2.2 Å / Lowest resolution: 2.3 Å / Rmerge(I) obs: 0.47 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1D8D Resolution: 2.2→50 Å / Rfactor Rfree error: 0.004 / Data cutoff high absF: 10000000 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 2 / Stereochemistry target values: Engh & Huber / Details: Bulk solvent model used
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 34.5 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→50 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.2→2.3 Å / Rfactor Rfree error: 0.015 / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS σ(F): 2 / % reflection Rfree: 5 % / Rfactor obs: 0.16 / Rfactor Rwork: 0.16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 34.5 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.255 / % reflection Rfree: 5.1 % / Rfactor Rwork: 0.237 / Rfactor obs: 0.237 |
 Movie
Movie Controller
Controller
















 PDBj
PDBj







