+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1hha | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Decaplanin first P6122-Form | ||||||||||||
 Components Components | DECAPLANIN | ||||||||||||
 Keywords Keywords | ANTIBIOTIC / GLYCOPEPTIDE | ||||||||||||
| Function / homology | Decaplanin / 4-epi-vancosamine / :  Function and homology information Function and homology information | ||||||||||||
| Biological species |  UNCULTURED ACTINOMYCETE (environmental samples) UNCULTURED ACTINOMYCETE (environmental samples) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 1.9 Å MAD / Resolution: 1.9 Å | ||||||||||||
 Authors Authors | Lehmann, C. / Vertessy, L. / Sheldrick, G.M. / Dauter, Z. / Dauter, M. | ||||||||||||
 Citation Citation |  Journal: Helv.Chim.Acta / Year: 2003 Journal: Helv.Chim.Acta / Year: 2003Title: Structures of Four Crystal Forms of Decaplanin Authors: Lehmann, C. / Debreczeni, J.E. / Bunkoczi, G. / Dauter, M. / Dauter, Z. / Vertesy, L. / Sheldrick, G.M. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1hha.cif.gz 1hha.cif.gz | 26.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1hha.ent.gz pdb1hha.ent.gz | 19.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1hha.json.gz 1hha.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hh/1hha https://data.pdbj.org/pub/pdb/validation_reports/hh/1hha ftp://data.pdbj.org/pub/pdb/validation_reports/hh/1hha ftp://data.pdbj.org/pub/pdb/validation_reports/hh/1hha | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 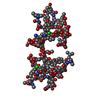
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 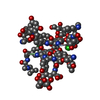
| ||||||||||||||||
| 2 | 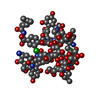
| ||||||||||||||||
| Unit cell |
| ||||||||||||||||
| Components on special symmetry positions |
| ||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
| #1: Protein/peptide | | #2: Polysaccharide | alpha-L-rhamnopyranose-(1-2)-beta-D-glucopyranose 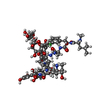  Source method: isolated from a genetically manipulated source Details: DECAPLANIN IS A TRICYCLIC GLYCOPEPTIDE. THE SCAFFOLD IS A HEPTAPEPTIDE WITH THE CONFIGURATION D-D-L-D-D-L-L, GLYCOSYLATED References: Decaplanin #3: Sugar | ChemComp-ERE / 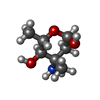  Type: L-saccharide, alpha linking, Glycopeptide / Class: Antibiotic / Mass: 161.199 Da / Num. of mol.: 4 Type: L-saccharide, alpha linking, Glycopeptide / Class: Antibiotic / Mass: 161.199 Da / Num. of mol.: 4Source method: isolated from a genetically manipulated source Formula: C7H15NO3 Details: DECAPLANIN IS A TRICYCLIC GLYCOPEPTIDE. THE SCAFFOLD IS A HEPTAPEPTIDE WITH THE CONFIGURATION D-D-L-D-D-L-L, GLYCOSYLATED References: Decaplanin #4: Chemical | #5: Water | ChemComp-HOH / | Compound details | DECAPLANIN IS A TRICYCLIC GLYCOPEPTIDE. HERE, DECAPLANIN IS REPRESENTED BY GROUPING TOGETHER THE ...DECAPLANIN | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.93 Å3/Da / Density % sol: 57.61 % |
|---|---|
| Crystal grow | pH: 8.5 / Details: 0.1M TRIS, PH=8.5, 44% MGSO4, pH 8.50 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X9B / Wavelength: 0.98000,0.98500 / Beamline: X9B / Wavelength: 0.98000,0.98500 | |||||||||
| Detector | Type:  CHESS CHESS  / Detector: CCD / Date: Oct 15, 1999 / Detector: CCD / Date: Oct 15, 1999 | |||||||||
| Radiation | Protocol: MAD / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||
| Radiation wavelength |
| |||||||||
| Reflection | Resolution: 1.9→23.23 Å / Num. obs: 8592 / % possible obs: 99.5 % / Redundancy: 10.5 % / Rmerge(I) obs: 0.0382 / Net I/σ(I): 26.2 | |||||||||
| Reflection shell | Resolution: 1.9→2 Å / Redundancy: 10.7 % / Rmerge(I) obs: 0.1031 / Mean I/σ(I) obs: 7.28 / % possible all: 99.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MAD / Resolution: 1.9→23.23 Å / Num. parameters: 2176 / Num. restraintsaints: 1843 / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: ENGH AND HUBER MAD / Resolution: 1.9→23.23 Å / Num. parameters: 2176 / Num. restraintsaints: 1843 / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: ENGH AND HUBER
| |||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: MOEWS & KRETSINGER, J.MOL.BIOL.91(1973)201-228 | |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 9 / Occupancy sum hydrogen: 432.9 / Occupancy sum non hydrogen: 521.8 | |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→23.23 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


