[English] 日本語
 Yorodumi
Yorodumi- PDB-1hes: MU2 ADAPTIN SUBUNIT (AP50) OF AP2 ADAPTOR (SECOND DOMAIN), COMPLE... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1hes | ||||||
|---|---|---|---|---|---|---|---|
| Title | MU2 ADAPTIN SUBUNIT (AP50) OF AP2 ADAPTOR (SECOND DOMAIN), COMPLEXED WITH P-selectin INTERNALIZATION PEPTIDE SHLGTYGVFTNAA | ||||||
 Components Components |
| ||||||
 Keywords Keywords | ENDOCYTOSIS/EXOCYTOSIS / ENDOCYTOSIS / ADAPTOR / PEPTIDE COMPLEX / PEPTIDE BINDING PROTE / ENDOCYTOSIS-EXOCYTOSIS complex | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of integrin activation / Gap junction degradation / Formation of annular gap junctions / fucose binding / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / LDL clearance / Retrograde neurotrophin signalling / VLDLR internalisation and degradation / WNT5A-dependent internalization of FZD4 / glycosphingolipid binding ...regulation of integrin activation / Gap junction degradation / Formation of annular gap junctions / fucose binding / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / LDL clearance / Retrograde neurotrophin signalling / VLDLR internalisation and degradation / WNT5A-dependent internalization of FZD4 / glycosphingolipid binding / platelet dense granule membrane / positive regulation of leukocyte tethering or rolling / extrinsic component of presynaptic endocytic zone membrane / MHC class II antigen presentation / sialic acid binding / regulation of vesicle size / oligosaccharide binding / AP-2 adaptor complex / postsynaptic neurotransmitter receptor internalization / Recycling pathway of L1 / positive regulation of synaptic vesicle endocytosis / Cargo recognition for clathrin-mediated endocytosis / clathrin-cargo adaptor activity / calcium-dependent cell-cell adhesion / Clathrin-mediated endocytosis / platelet alpha granule membrane / vesicle budding from membrane / clathrin-dependent endocytosis / leukocyte tethering or rolling / signal sequence binding / positive regulation of platelet activation / positive regulation of leukocyte migration / heterophilic cell-cell adhesion / low-density lipoprotein particle receptor binding / leukocyte cell-cell adhesion / Trafficking of GluR2-containing AMPA receptors / positive regulation of receptor internalization / synaptic vesicle endocytosis / negative regulation of protein localization to plasma membrane / vesicle-mediated transport / response to cytokine / clathrin-coated pit / Cell surface interactions at the vascular wall / intracellular protein transport / lipopolysaccharide binding / cell-cell adhesion / receptor internalization / integrin binding / endocytosis / terminal bouton / disordered domain specific binding / calcium-dependent protein binding / synaptic vesicle / Platelet degranulation / heparin binding / protein-containing complex assembly / cytoplasmic vesicle / response to lipopolysaccharide / defense response to Gram-negative bacterium / transmembrane transporter binding / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / cell adhesion / postsynapse / inflammatory response / external side of plasma membrane / calcium ion binding / synapse / lipid binding / glutamatergic synapse / extracellular space / plasma membrane Similarity search - Function | ||||||
| Biological species |   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3 Å MOLECULAR REPLACEMENT / Resolution: 3 Å | ||||||
 Authors Authors | Owen, D.J. / Evans, P.R. / Green, S.A. | ||||||
 Citation Citation |  Journal: Traffic / Year: 2001 Journal: Traffic / Year: 2001Title: A Third Specificity-Determining Site in Mu 2 Adaptin for Sequences Upstream of Yxx Phi Sorting Motifs Authors: Owen, D.J. / Setiadi, H. / Evans, P.R. / Mcever, R.P. / Green, S.A. #1:  Journal: Science / Year: 1998 Journal: Science / Year: 1998Title: A Structural Explanation for the Recognition of Tyrosine-Based Endocytotic Signals Authors: Owen, D.J. / Evans, P.R. | ||||||
| History |
| ||||||
| Remark 700 | SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN ... SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE SHEET RECORDS BELOW, TWO SHEETS ARE DEFINED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1hes.cif.gz 1hes.cif.gz | 67.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1hes.ent.gz pdb1hes.ent.gz | 49.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1hes.json.gz 1hes.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/he/1hes https://data.pdbj.org/pub/pdb/validation_reports/he/1hes ftp://data.pdbj.org/pub/pdb/validation_reports/he/1hes ftp://data.pdbj.org/pub/pdb/validation_reports/he/1hes | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1bw8S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 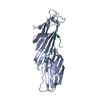
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 32758.363 Da / Num. of mol.: 1 / Fragment: INTERNALIZATION SIGNAL BINDING DOMAIN Source method: isolated from a genetically manipulated source Details: THE FIRST SEVEN RESIDUES OF THE POLYMER MAKE UP A HIS-TAG Source: (gene. exp.)   |
|---|---|
| #2: Protein/peptide | Mass: 1882.015 Da / Num. of mol.: 1 / Fragment: INTERNALIZATION SIGNAL / Source method: obtained synthetically Details: PEPTIDE INTERNALISATION SIGNAL MOTIF FROM P-SELECTIN SHLGTYGVFTNAAFDPSP Source: (synth.)  HOMO SAPIENS (human) / References: UniProt: P16109 HOMO SAPIENS (human) / References: UniProt: P16109 |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 5.7 Å3/Da / Density % sol: 79 % / Description: ISOMORPHOUS TO 1BW8 | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, hanging drop / pH: 7.1 Details: HANGING DROP, 2.2M NACL, 0.4M NA/K PHOSPHATE, 10MM DTT 0.1M MES PH 7.1, 15% GLYCEROL, 16 DEGREES, MOLAR RATIO OF PEPTIDE TO PROTEIN 3:1 | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 16 ℃ / Method: vapor diffusion, hanging drop / Details: Owen, D.J., (1998) Science, 282, 1327. / PH range low: 7.1 / PH range high: 6.5 | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SRS SRS  / Beamline: PX9.6 / Wavelength: 0.877 / Beamline: PX9.6 / Wavelength: 0.877 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Feb 18, 2000 / Details: MIRROR |
| Radiation | Monochromator: YES / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.877 Å / Relative weight: 1 |
| Reflection | Resolution: 3→22 Å / Num. obs: 13729 / % possible obs: 100 % / Observed criterion σ(I): 4 / Redundancy: 9.2 % / Biso Wilson estimate: 90 Å2 / Rmerge(I) obs: 0.146 / Rsym value: 0.146 / Net I/σ(I): 17.4 |
| Reflection shell | Resolution: 3→3.16 Å / Redundancy: 14.7 % / Rmerge(I) obs: 0.0106 / Mean I/σ(I) obs: 2 / Rsym value: 0.0106 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1BW8 Resolution: 3→100 Å / SU B: 18.91 / SU ML: 0.359 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.442 / ESU R Free: 0.329 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||
| Displacement parameters | Biso mean: 51 Å2
| ||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3→100 Å
| ||||||||||||||||||||
| Software | *PLUS Name: REFMAC / Version: 5 / Classification: refinement | ||||||||||||||||||||
| Refinement | *PLUS Rfactor Rwork: 0.21 | ||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj












