[English] 日本語
 Yorodumi
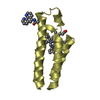
Yorodumi- PDB-1h3o: Crystal Structure of the Human TAF4-TAF12 (TAFII135-TAFII20) Complex -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1h3o | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the Human TAF4-TAF12 (TAFII135-TAFII20) Complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION/TBP-ASSOCIATED FACTORS / TBP-ASSOCIATED FACTORS / TFIID / RNA POLYMERASE II TRANSCRIPTION / HISTONE FOLD DOMAINS / NUCLEAR PROTEIN / TRANSCRIPTION-TBP-ASSOCIATED FACTORS complex | ||||||
| Function / homology |  Function and homology information Function and homology informationtranscription factor TFTC complex / SAGA complex / transcription factor TFIID complex / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance ...transcription factor TFTC complex / SAGA complex / transcription factor TFIID complex / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / regulation of RNA splicing / aryl hydrocarbon receptor binding / MLL1 complex / positive regulation of transcription initiation by RNA polymerase II / regulation of DNA repair / RNA polymerase II preinitiation complex assembly / ovarian follicle development / RNA Polymerase II Pre-transcription Events / TBP-class protein binding / male germ cell nucleus / transcription initiation at RNA polymerase II promoter / DNA-templated transcription initiation / mRNA transcription by RNA polymerase II / HATs acetylate histones / Regulation of TP53 Activity through Phosphorylation / DNA-binding transcription factor binding / transcription by RNA polymerase II / transcription coactivator activity / protein heterodimerization activity / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / chromatin / positive regulation of DNA-templated transcription / positive regulation of transcription by RNA polymerase II / protein-containing complex / DNA binding / nucleoplasm / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 2.3 Å MAD / Resolution: 2.3 Å | ||||||
 Authors Authors | Werten, S. / Mitschler, A. / Moras, D. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2002 Journal: J.Biol.Chem. / Year: 2002Title: Crystal Structure of a Subcomplex of Human Transcription Factor TFIID Formed by TATA Binding Protein-Associated Factors Htaf4 (Htaf(II)135) and Htaf12 (Htaf(II)20). Authors: Werten, S. / Mitschler, A. / Romier, C. / Gangloff, Y.-G. / Thuault, S. / Davidson, I. / Moras, D. #1: Journal: Mol.Cell.Biol. / Year: 2000 Title: The Human TFIID Components Tafii135 and Tafii20 and the Yeast Saga Components Ada1 and Tafii68 Heterodimerize to Form Histone-Like Pairs Authors: Gangloff, Y.-G. / Werten, S. / Romier, C. / Carre, L. / Poch, O. / Moras, D. / Davidson, I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1h3o.cif.gz 1h3o.cif.gz | 67 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1h3o.ent.gz pdb1h3o.ent.gz | 50.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1h3o.json.gz 1h3o.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h3/1h3o https://data.pdbj.org/pub/pdb/validation_reports/h3/1h3o ftp://data.pdbj.org/pub/pdb/validation_reports/h3/1h3o ftp://data.pdbj.org/pub/pdb/validation_reports/h3/1h3o | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (0.96526, -0.00695, 0.26119), Vector: Details | TWO HETERODIMERS FORMED BY CHAINS A AND B , OR CHAINSC AND D. | |
- Components
Components
| #1: Protein | Mass: 8813.803 Da / Num. of mol.: 2 / Fragment: HISTONE FOLD DOMAIN, RESIDUES 870-943 / Mutation: YES Source method: isolated from a genetically manipulated source Details: N-TERMINAL SELENOMETHIONINE INSERT, UNIFORM SELENO-METHIONINE LABELING Source: (gene. exp.)  HOMO SAPIENS (human) / Description: SYNTHETIC GENE / Plasmid: PACYC-11B / Production host: HOMO SAPIENS (human) / Description: SYNTHETIC GENE / Plasmid: PACYC-11B / Production host:  #2: Protein | Mass: 9053.836 Da / Num. of mol.: 2 / Fragment: HISTONE FOLD DOMAIN, RESIDUES 57-128 / Mutation: YES Source method: isolated from a genetically manipulated source Details: RESIDUES (GLY SER HIS MSE) INSERTED AT THE N-TERMINUS, REMAINDER OF HISTIDINE-TAG AFTER THROMBIN TREATMENT, UNIFORM SELENO-METHIONINE LABELING Source: (gene. exp.)  HOMO SAPIENS (human) / Description: SYNTHETIC GENE / Plasmid: PET-15B / Production host: HOMO SAPIENS (human) / Description: SYNTHETIC GENE / Plasmid: PET-15B / Production host:  #3: Water | ChemComp-HOH / | Compound details | HTAF4: MULTIMERIC PROTEIN COMPLEX THAT PLAYS A CENTRAL ROLE IN MEDIATING PROMOTER RESPONSES TO ...HTAF4: MULTIMERIC | Has protein modification | Y | Sequence details | ENGINEERED DELETION MUTANT THE HTAF12 POLYPEPTIDE THAT WAS USED FOR CRYSTALLIZATION CONTAINED THE ...ENGINEERED | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 42 % Description: DATA QUALITY STATISTICS LISTED PERTAIN TO REMOTE WAVELENGTH DATA (0.90730 A) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 5.25 / Details: pH 5.25 | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 24 ℃ / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: BW7A / Wavelength: 0.90730,0.97740 / Beamline: BW7A / Wavelength: 0.90730,0.97740 | |||||||||
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Jun 9, 2001 / Details: PREMIRROR, BENT MIRROR | |||||||||
| Radiation | Monochromator: DOUBLE CRYSTAL FOCUSSING MONOCHROMATOR / Protocol: MAD / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||
| Radiation wavelength |
| |||||||||
| Reflection | Resolution: 2.3→20 Å / Num. obs: 24237 / % possible obs: 92.3 % / Redundancy: 1.5 % / Biso Wilson estimate: 21.9 Å2 / Rsym value: 0.034 / Net I/σ(I): 19.9 | |||||||||
| Reflection shell | Resolution: 2.3→2.38 Å / Redundancy: 1.5 % / Mean I/σ(I) obs: 4.45 / Rsym value: 0.167 / % possible all: 90.1 | |||||||||
| Reflection | *PLUS Highest resolution: 2.3 Å / Num. obs: 25898 / % possible obs: 98.9 % / Num. measured all: 61754 / Rmerge(I) obs: 0.035 | |||||||||
| Reflection shell | *PLUS % possible obs: 99.7 % / Num. unique obs: 2588 / Num. measured obs: 6073 / Rmerge(I) obs: 0.122 / Mean I/σ(I) obs: 7.56 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MAD / Resolution: 2.3→19.63 Å / Rfactor Rfree error: 0.011 / Data cutoff high absF: 1965882.6 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: MLF MAD / Resolution: 2.3→19.63 Å / Rfactor Rfree error: 0.011 / Data cutoff high absF: 1965882.6 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: MLFDetails: NO ELECTRON DENSITY WAS OBSERVED FOR THE C-TERMINAL PORTION OF HTAF4, RESIDUES 918-943, WHICH IS THEREFORE ABSENT FROM THE MODEL. THE FACT THAT PART OF THE PROTEIN COMPLEX DID NOT SHOW UP IN ...Details: NO ELECTRON DENSITY WAS OBSERVED FOR THE C-TERMINAL PORTION OF HTAF4, RESIDUES 918-943, WHICH IS THEREFORE ABSENT FROM THE MODEL. THE FACT THAT PART OF THE PROTEIN COMPLEX DID NOT SHOW UP IN ELECTRON DENSITY MAPS WAS NOT DUE TO PROTEOLYSIS (AS EVIDENCED BY MASS SPECTROSCOPY OF REDISSOLVED CRYSTALS), INDICATING THAT THE REGIONS INVOLVED ARE DISORDERED WITHIN THE CRYSTAL LATTICE.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 58.9338 Å2 / ksol: 0.357292 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 47.4 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→19.63 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.3→2.44 Å / Rfactor Rfree error: 0.035 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 20 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj


