[English] 日本語
 Yorodumi
Yorodumi- PDB-1fv0: FIRST STRUCTURAL EVIDENCE OF THE INHIBITION OF PHOSPHOLIPASE A2 B... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1fv0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | FIRST STRUCTURAL EVIDENCE OF THE INHIBITION OF PHOSPHOLIPASE A2 BY ARISTOLOCHIC ACID: CRYSTAL STRUCTURE OF A COMPLEX FORMED BETWEEN PHOSPHOLIPASE A2 AND ARISTOLOCHIC ACID | ||||||
 Components Components | PHOSPHOLIPASE A2 | ||||||
 Keywords Keywords | TOXIN / phospholipase A2 / Daboia russelli pulchella / neurotoxic / edema | ||||||
| Function / homology |  Function and homology information Function and homology informationphospholipase A2 / : / arachidonate secretion / lipid catabolic process / negative regulation of T cell proliferation / phospholipid metabolic process / phospholipid binding / toxin activity / calcium ion binding / extracellular region Similarity search - Function | ||||||
| Biological species |  Daboia russellii pulchella (snake) Daboia russellii pulchella (snake) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.7 Å MOLECULAR REPLACEMENT / Resolution: 1.7 Å | ||||||
 Authors Authors | Chandra, V. / Jasti, J. / Kaur, P. / Srinivasan, A. / Betzel, C. / Singh, T.P. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2002 Journal: Biochemistry / Year: 2002Title: Structural Basis of Phospholipase A2 Inhibition for the Synthesis of Prostaglandins by the Plant Alkaloid Aristolochic Acid from a 1.7 A Crystal Structure Authors: Chandra, V. / Jasti, J. / Kaur, P. / Srinivasan, A. / Betzel, C. / Singh, T.P. #1:  Journal: To be Published Journal: To be PublishedTitle: First structural evidence of antiinflammatory action of vitamin E (2,5,7,8-tetramethyl-2-(4',8',12'-trimethyltridecyl)-6-chromanol) through its binding to phospholipase A2 specifically: ...Title: First structural evidence of antiinflammatory action of vitamin E (2,5,7,8-tetramethyl-2-(4',8',12'-trimethyltridecyl)-6-chromanol) through its binding to phospholipase A2 specifically: Crystal structure of a complex formed between phospholipase A2 and vitamin E at 1.80 resolution Authors: Chandra, V. / Jasti, J. / Kaur, P. / Perbandt, M. / Betzel, C.H. / Singh, T.P. #2:  Journal: J.Mol.Biol. / Year: 2000 Journal: J.Mol.Biol. / Year: 2000Title: Three-dimensional structure of a presynaptic neurotoxic phospholipase A2 from Daboia russelli pulchella at 2.4 resolution Authors: Chandra, V. / Kaur, P. / Srinivasan, A. / Singh, T.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1fv0.cif.gz 1fv0.cif.gz | 70.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1fv0.ent.gz pdb1fv0.ent.gz | 51.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1fv0.json.gz 1fv0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fv/1fv0 https://data.pdbj.org/pub/pdb/validation_reports/fv/1fv0 ftp://data.pdbj.org/pub/pdb/validation_reports/fv/1fv0 ftp://data.pdbj.org/pub/pdb/validation_reports/fv/1fv0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1cl5S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| 3 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 13629.767 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Daboia russellii pulchella (snake) / Secretion: VENOM / Species: Daboia russellii / Strain: pulchella / References: UniProt: P59071 Daboia russellii pulchella (snake) / Secretion: VENOM / Species: Daboia russellii / Strain: pulchella / References: UniProt: P59071 |
|---|
-Non-polymers , 6 types, 325 molecules 

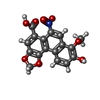

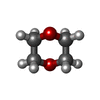






| #2: Chemical | ChemComp-SO4 / #3: Chemical | ChemComp-ACT / | #4: Chemical | ChemComp-9AR / | #5: Chemical | #6: Chemical | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.53 Å3/Da / Density % sol: 50.97 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 20mM Sodium cacodylate, 1.4M Ammonium sulfate, 4mM Calcium chloride, 3% dioxane, pH 6.5, VAPOR DIFFUSION, HANGING DROP, temperature 298K | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 7 | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 180 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: X11 / Wavelength: 0.98 / Beamline: X11 / Wavelength: 0.98 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Jun 20, 1999 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.98 Å / Relative weight: 1 |
| Reflection | Resolution: 1.7→30 Å / Num. all: 29231 / Num. obs: 29231 / % possible obs: 99 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 6.6 % / Biso Wilson estimate: 25.5 Å2 / Rmerge(I) obs: 0.045 / Rsym value: 0.045 / Net I/σ(I): 10.2 |
| Reflection shell | Resolution: 1.7→1.73 Å / Redundancy: 2.5 % / Rmerge(I) obs: 0.21 / Mean I/σ(I) obs: 4.2 / Rsym value: 0.182 / % possible all: 97.9 |
| Reflection | *PLUS Lowest resolution: 20 Å / Num. obs: 28922 / % possible obs: 99.1 % / Num. measured all: 419758 |
| Reflection shell | *PLUS % possible obs: 97.9 % / Rmerge(I) obs: 0.034 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1CL5 Resolution: 1.7→20 Å / Rfactor Rfree error: 0.007 / Data cutoff high absF: 1442367.33 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber / Details: Used weighted full matrix least squares procedure.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 60.8357 Å2 / ksol: 0.424778 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27.9 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.7→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.7→1.81 Å / Rfactor Rfree error: 0.017 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 1.7 Å / Lowest resolution: 20 Å / % reflection Rfree: 5 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj






