[English] 日本語
 Yorodumi
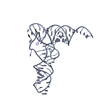
Yorodumi- PDB-1fir: CRYSTAL STRUCTURE OF HIV-1 REVERSE TRANSCRIPTION PRIMER TRNA(LYS3) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1fir | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF HIV-1 REVERSE TRANSCRIPTION PRIMER TRNA(LYS3) | ||||||
 Components Components | HIV-1 REVERSE TRANSCRIPTION PRIMER TRNA(LYS3) | ||||||
 Keywords Keywords | RNA / mammalian transfert ribonucleic acid / HIV-1 primer tRNA / amino acid transport / canonical tRNA structure / canonical anticodon / frameshifting / codon/anticodon mimicry / modified bases | ||||||
| Function / homology | RNA / RNA (> 10) Function and homology information Function and homology information | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.3 Å MOLECULAR REPLACEMENT / Resolution: 3.3 Å | ||||||
 Authors Authors | Benas, P. / Dumas, P. | ||||||
 Citation Citation |  Journal: RNA / Year: 2000 Journal: RNA / Year: 2000Title: The crystal structure of HIV reverse-transcription primer tRNA(Lys,3) shows a canonical anticodon loop. Authors: Benas, P. / Bec, G. / Keith, G. / Marquet, R. / Ehresmann, C. / Ehresmann, B. / Dumas, P. #1:  Journal: To be Published / Year: 2000 Journal: To be Published / Year: 2000Title: Molecular blocs in tRNAs that could prevent +1 frameshifting Authors: Benas, P. / Dumas, P. #2:  Journal: Thesis / Year: 2000 Journal: Thesis / Year: 2000Title: Studies on the ternary initiation complex of HIV-1 reverse transcription and crystallographic structures of two partners Authors: Benas, P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1fir.cif.gz 1fir.cif.gz | 53.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1fir.ent.gz pdb1fir.ent.gz | 42.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1fir.json.gz 1fir.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fi/1fir https://data.pdbj.org/pub/pdb/validation_reports/fi/1fir ftp://data.pdbj.org/pub/pdb/validation_reports/fi/1fir ftp://data.pdbj.org/pub/pdb/validation_reports/fi/1fir | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4tnaS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
| #1: RNA chain | Mass: 24847.088 Da / Num. of mol.: 1 / Source method: isolated from a natural source Details: THIS SEQUENCE OCCURS NATURALLY IN HUMANS, BOVINES AND CHICKENS Source: (natural)  |
|---|---|
| #2: Chemical | ChemComp-MG / |
| #3: Chemical | ChemComp-NA / |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 5.12 Å3/Da / Density % sol: 78.73 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 5.6 Details: 50 mM MES-KOH pH 5.6, 1.6 to 1.8 M Li2SO4, 10 mM MgCl2, pH 5.6, VAPOR DIFFUSION, HANGING DROP, temperature 293K | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions |
| ||||||||||||||||||||||||||||||||||||||||||
| Crystal | *PLUS Density % sol: 78.7 % | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / Method: vapor diffusion | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 110 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM02 / Wavelength: 0.9805 / Beamline: BM02 / Wavelength: 0.9805 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Feb 2, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9805 Å / Relative weight: 1 |
| Reflection | Resolution: 3.3→40 Å / Num. all: 6482 / Num. obs: 6482 / % possible obs: 88.2 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.2 % / Rsym value: 0.081 / Net I/σ(I): 10.5 |
| Reflection shell | Resolution: 3.3→3.42 Å / Redundancy: 2.2 % / Mean I/σ(I) obs: 4.3 / Num. unique all: 503 / Rsym value: 0.165 / % possible all: 71.6 |
| Reflection | *PLUS Rmerge(I) obs: 0.081 |
| Reflection shell | *PLUS % possible obs: 71.6 % / Rmerge(I) obs: 0.165 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4TNA Resolution: 3.3→40 Å / Rfactor Rfree error: 0.01 / Data cutoff high absF: 836049.77 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 3 Stereochemistry target values: modified CNS libraries (Parkinson et al.) Details: R and free R improved by 12% and 11% respectively by a proper mask definition (see primary ref.)
| |||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 34.19 Å2 / ksol: 0.03 e/Å3 | |||||||||||||||||||||||||
| Displacement parameters | Biso mean: 33.2 Å2
| |||||||||||||||||||||||||
| Refine analyze |
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.3→40 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.3→3.51 Å / Rfactor Rfree error: 0.04 / Total num. of bins used: 6
| |||||||||||||||||||||||||
| Xplor file |
| |||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 0.4 / Classification: refinement | |||||||||||||||||||||||||
| Refinement | *PLUS σ(F): 3 / % reflection Rfree: 9.2 % | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 33.2 Å2 | |||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| |||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.234 / % reflection Rfree: 4.9 % / Rfactor Rwork: 0.221 |
 Movie
Movie Controller
Controller











 PDBj
PDBj


































