[English] 日本語
 Yorodumi
Yorodumi- PDB-1ets: REFINED 2.3 ANGSTROMS X-RAY CRYSTAL STRUCTURE OF BOVINE THROMBIN ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ets | ||||||
|---|---|---|---|---|---|---|---|
| Title | REFINED 2.3 ANGSTROMS X-RAY CRYSTAL STRUCTURE OF BOVINE THROMBIN COMPLEXES FORMED WITH THE BENZAMIDINE AND ARGININE-BASED THROMBIN INHIBITORS NAPAP, 4-TAPAP AND MQPA: A STARTING POINT FOR IMPROVING ANTITHROMBOTICS | ||||||
 Components Components | (EPSILON-THROMBIN) x 2 | ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE inhibitor / SERINE PROTEINASE / HYDROLASE-HYDROLASE INHIBITOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationfibrinogen binding / thrombin / protein polymerization / positive regulation of blood coagulation / acute-phase response / platelet activation / : / serine-type endopeptidase activity / calcium ion binding / proteolysis / extracellular space Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.3 Å X-RAY DIFFRACTION / Resolution: 2.3 Å | ||||||
 Authors Authors | Bode, W. / Brandstetter, H. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1992 Journal: J.Mol.Biol. / Year: 1992Title: Refined 2.3 A X-ray crystal structure of bovine thrombin complexes formed with the benzamidine and arginine-based thrombin inhibitors NAPAP, 4-TAPAP and MQPA. A starting point for improving antithrombotics. Authors: Brandstetter, H. / Turk, D. / Hoeffken, H.W. / Grosse, D. / Sturzebecher, J. / Martin, P.D. / Edwards, B.F. / Bode, W. #1:  Journal: To be Published Journal: To be PublishedTitle: Crystallographic Determination of Thrombin Complexes with Small Synthetic Inhibitors as a Starting Point for the Receptor-Based Design of Antithrombotics Authors: Bode, W. / Brandstetter, H. / Turk, D. / Bauer, M. / Stuerzebecher, J. #2:  Journal: To be Published Journal: To be PublishedTitle: X-Ray Crystal Structure of Thrombin in Complex with D-Phe-Pro-Arg and with Small Benzamidine and Arginine-Based "Non-Peptidic" Inhibitors Authors: Bode, W. #3:  Journal: Protein Sci. / Year: 1992 Journal: Protein Sci. / Year: 1992Title: The Refined 1.9-Angstroms X-Ray Crystal Structure of D-Phe-Pro-Arg Chloromethylketone-Inhibited Human Alpha-Thrombin: Structure Analysis, Overall Structure, Electrostatic Properties, Detailed ...Title: The Refined 1.9-Angstroms X-Ray Crystal Structure of D-Phe-Pro-Arg Chloromethylketone-Inhibited Human Alpha-Thrombin: Structure Analysis, Overall Structure, Electrostatic Properties, Detailed Active-Site Geometry, and Structure-Function Relationships Authors: Bode, W. / Turk, D. / Karshikov, A. #4:  Journal: FEBS Lett. / Year: 1991 Journal: FEBS Lett. / Year: 1991Title: Geometry of Binding of the Nalpha-Tosylated Piperidides of M-Amidino-, P-Amidino-and P-Guanidino Phenylalanine to Thrombin and Trypsin: X-Ray Crystal Structures of Their Trypsin Complexes and ...Title: Geometry of Binding of the Nalpha-Tosylated Piperidides of M-Amidino-, P-Amidino-and P-Guanidino Phenylalanine to Thrombin and Trypsin: X-Ray Crystal Structures of Their Trypsin Complexes and Modeling of Their Thrombin Complexes Authors: Turk, D. / Stuerzebecher, J. / Bode, W. #5:  Journal: Eur.J.Biochem. / Year: 1990 Journal: Eur.J.Biochem. / Year: 1990Title: Geometry of Binding of the Benzamidine-and Arginine-Based Inhibitors N-Alpha-(2-Naphthyl-Sulphonyl-Glycyl)-Dl-P-Amidinophenylalanyl-Piperidine (Napap) and (2R,4R)-4-Methyl-1-[N-Alpha-(3-Methyl- ...Title: Geometry of Binding of the Benzamidine-and Arginine-Based Inhibitors N-Alpha-(2-Naphthyl-Sulphonyl-Glycyl)-Dl-P-Amidinophenylalanyl-Piperidine (Napap) and (2R,4R)-4-Methyl-1-[N-Alpha-(3-Methyl-1,2,3,4-Tetrahydro-8-Quinolinesulphonyl)-L-Arginyl]-2-Piperidine Carboxylic Acid (Mqpa) to Human Alpha-Thrombin: X-Ray Crystallographic Determination of the Napap-Trypsin Complex and Modeling of Napap-Thrombin and Mqpa-Thrombin Authors: Bode, W. / Turk, D. / Stuerzebecher, J. #6:  Journal: Embo J. / Year: 1989 Journal: Embo J. / Year: 1989Title: The Refined 1.9 Angstroms Crystal Structure of Human Alpha-Thrombin: Interaction with D-Phe-Pro-Arg Chloromethylketone and Significance of the Tyr-Pro-Pro-Trp Insertion Segment Authors: Bode, W. / Mayr, I. / Baumann, U. / Huber, R. / Stone, S.R. / Hofsteenge, J. | ||||||
| History |
| ||||||
| Remark 700 | SHEET THE SHEETS PRESENTED AS *S1* AND *S2* ON SHEET RECORDS BELOW ARE ACTUALLY SIX-STRANDED BETA- ...SHEET THE SHEETS PRESENTED AS *S1* AND *S2* ON SHEET RECORDS BELOW ARE ACTUALLY SIX-STRANDED BETA-BARRELS. THESE ARE REPRESENTED BY SEVEN-STRANDED SHEETS IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. |
- Structure visualization
Structure visualization
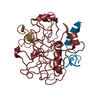
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ets.cif.gz 1ets.cif.gz | 101.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ets.ent.gz pdb1ets.ent.gz | 78.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ets.json.gz 1ets.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/et/1ets https://data.pdbj.org/pub/pdb/validation_reports/et/1ets ftp://data.pdbj.org/pub/pdb/validation_reports/et/1ets ftp://data.pdbj.org/pub/pdb/validation_reports/et/1ets | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: RESIDUE PRO H 37 IS A CIS PROLINE. |
- Components
Components
| #1: Protein/peptide | Mass: 5735.240 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
|---|---|
| #2: Protein | Mass: 29772.422 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
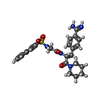
| #3: Chemical | ChemComp-MID / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
| Nonpolymer details | AMIDINOPHENYLALANINE (OF NAPAP) BINDS TO S1 SPECIFICITY POCKET, PIPERIDINE TO S2 AND NAPHLYL GROUP ...AMIDINOPHE |
| Sequence details | THE NUMBERING OF THROMBIN RESIDUES IS BASED ON TOPOLOGICA |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.85 Å3/Da / Density % sol: 56.85 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 8 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 2.3 Å / Num. obs: 13578 / % possible obs: 78 % / Observed criterion σ(I): 2 / Num. measured all: 84833 / Rmerge(I) obs: 0.142 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.3→8 Å / Rfactor Rwork: 0.178 / Rfactor obs: 0.178 Details: THERE IS A CLEAVAGE IN THE CHAIN AT THR 149A - SER 149B IN THE INSERTION LOOP OF THROMBIN. THE AUTHORS DO NOT SEE THIS CLEAVAGE DUE TO DISORDER AT BOTH FLANKING REGIONS. NOTE THAT THE ...Details: THERE IS A CLEAVAGE IN THE CHAIN AT THR 149A - SER 149B IN THE INSERTION LOOP OF THROMBIN. THE AUTHORS DO NOT SEE THIS CLEAVAGE DUE TO DISORDER AT BOTH FLANKING REGIONS. NOTE THAT THE OCCUPANCY OF RESIDUES 148 - 149E IS GIVEN AS 0.0 INDICATING THAT THEY WERE NOT SEEN IN THE ELECTRON DENSITY MAPS. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.3 Å / Lowest resolution: 8 Å / Num. reflection obs: 12503 / Rfactor obs: 0.178 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 40 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_d / Dev ideal: 3.089 |
 Movie
Movie Controller
Controller













 PDBj
PDBj