+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1e6c | ||||||
|---|---|---|---|---|---|---|---|
| Title | K15M MUTANT OF SHIKIMATE KINASE FROM ERWINIA CHRYSANTHEMI | ||||||
 Components Components | SHIKIMATE KINASE | ||||||
 Keywords Keywords | TRANSFERASE / MUTANT SHIKIMATE KINASE / PHOSPHORYL TRANSFER / ADP / SHIKIMATE PATHWAY / P-LOOP PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationshikimate kinase / shikimate kinase activity / chorismate biosynthetic process / aromatic amino acid family biosynthetic process / amino acid biosynthetic process / magnesium ion binding / ATP binding / cytosol Similarity search - Function | ||||||
| Biological species |  ERWINIA CHRYSANTHEMI (bacteria) ERWINIA CHRYSANTHEMI (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Maclean, J. / Krell, T. / Coggins, J.R. / Lapthorn, A.J. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 2001 Journal: Protein Sci. / Year: 2001Title: Biochemical and X-Ray Crystallographic Studies on Shikimate Kinase: The Important Structural Role of the P-Loop Lysine Authors: Krell, T. / Maclean, J. / Boam, D.J. / Cooper, A. / Resmini, M. / Brocklehurst, K. / Kelly, S.M. / Price, N.C. / Lapthorn, A.J. / Coggins, J.R. #1: Journal: J.Mol.Biol. / Year: 1998 Title: The Three-Dimensional Structure of Shikimate Kinase Authors: Krell, T. / Coggins, J.R. / Lapthorn, A.J. | ||||||
| History |
|
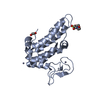
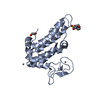
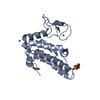
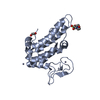
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1e6c.cif.gz 1e6c.cif.gz | 87 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1e6c.ent.gz pdb1e6c.ent.gz | 65.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1e6c.json.gz 1e6c.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e6/1e6c https://data.pdbj.org/pub/pdb/validation_reports/e6/1e6c ftp://data.pdbj.org/pub/pdb/validation_reports/e6/1e6c ftp://data.pdbj.org/pub/pdb/validation_reports/e6/1e6c | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1shkS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.50176, 0.00043, -0.86501), Vector: |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 18993.812 Da / Num. of mol.: 2 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  ERWINIA CHRYSANTHEMI (bacteria) / Gene: AROL / Plasmid: PTB361SK / Gene (production host): AROL / Production host: ERWINIA CHRYSANTHEMI (bacteria) / Gene: AROL / Plasmid: PTB361SK / Gene (production host): AROL / Production host:  |
|---|
-Non-polymers , 5 types, 224 molecules 








| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-MPD / ( #5: Chemical | ChemComp-MRD / ( | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Compound details | CHAIN A, B ENGINEERED MUTATION LYS15MET ENZYME REACTION: ATP + SHIKIMATE = ADP + SHIKIMATE 3- ...CHAIN A, B ENGINEERED |
|---|---|
| Sequence details | REFERENCE: THE SEQUENCE IS DESCRIBED IN MINTON N.P., WHITEHEAD P.J., ATKINSON T., GILBERT H.J. ...REFERENCE: THE SEQUENCE IS DESCRIBED IN MINTON N.P., WHITEHEAD P.J., ATKINSON T., GILBERT H.J. NUCLEIC ACIDS RES. 17:1769-1769(1989). |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.24 Å3/Da / Density % sol: 44.6 % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 8 Details: 10% PEG8000, 100MM TRIS/HCL BUFFER PH 8.0, 2.5MM ADP, 2.5MM SHIKIMATE, 10MM MGCL2 | ||||||||||||||||||||
| Crystal grow | *PLUS pH: 7.5 / Method: vapor diffusion, sitting drop | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SRS SRS  / Beamline: PX9.6 / Wavelength: 0.87 / Beamline: PX9.6 / Wavelength: 0.87 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Feb 15, 1998 / Details: MIRROR |
| Radiation | Monochromator: SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.87 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→21.4 Å / Num. obs: 27407 / % possible obs: 93 % / Observed criterion σ(I): 0 / Redundancy: 5.3 % / Biso Wilson estimate: 19.073 Å2 / Rmerge(I) obs: 0.078 / Rsym value: 0.078 / Net I/σ(I): 15 |
| Reflection shell | Resolution: 1.8→1.86 Å / Redundancy: 1.6 % / Rmerge(I) obs: 0.348 / Mean I/σ(I) obs: 1.9 / Rsym value: 0.348 / % possible all: 69.1 |
| Reflection | *PLUS Num. measured all: 164162 |
| Reflection shell | *PLUS Highest resolution: 1.8 Å / % possible obs: 69 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1SHK Resolution: 1.8→25 Å / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.16471 / ESU R Free: 0.14659 Details: HYDROGEN ATOM CONTRIBUTION AND EXTERNAL BULK SOLVENT CORRECTION WERE APPLIED AS FPART . THE SPACE GROUP ASSIGNMENT WAS EXTREMELY CLOSE BETWEEN P21 AND C2221. IT WAS DECIDED TO PROCEED WITH ...Details: HYDROGEN ATOM CONTRIBUTION AND EXTERNAL BULK SOLVENT CORRECTION WERE APPLIED AS FPART . THE SPACE GROUP ASSIGNMENT WAS EXTREMELY CLOSE BETWEEN P21 AND C2221. IT WAS DECIDED TO PROCEED WITH P21 AND USE STRICT NCS RESTRAINTS BETWEEN MONOMERS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 23.8 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→25 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: REFMAC / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.188 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller










 PDBj
PDBj



