[English] 日本語
 Yorodumi
Yorodumi- PDB-1dmy: COMPLEX BETWEEN MURINE MITOCHONDRIAL CARBONIC ANYHDRASE V AND THE... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1dmy | ||||||
|---|---|---|---|---|---|---|---|
| Title | COMPLEX BETWEEN MURINE MITOCHONDRIAL CARBONIC ANYHDRASE V AND THE TRANSITION STATE ANALOGUE ACETAZOLAMIDE | ||||||
 Components Components | MURINE CARBONIC ANHYDRASE V | ||||||
 Keywords Keywords | LYASE (OXO-ACID) / PROTON TRANSFER | ||||||
| Function / homology |  Function and homology information Function and homology informationReversible hydration of carbon dioxide / gluconeogenesis / carbonic anhydrase / carbonate dehydratase activity / mitochondrion / zinc ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.45 Å X-RAY DIFFRACTION / Resolution: 2.45 Å | ||||||
 Authors Authors | Boriack-Sjodin, P.A. / Christianson, D.W. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 1995 Journal: Proc.Natl.Acad.Sci.USA / Year: 1995Title: Structure determination of murine mitochondrial carbonic anhydrase V at 2.45-A resolution: implications for catalytic proton transfer and inhibitor design. Authors: Boriack-Sjodin, P.A. / Heck, R.W. / Laipis, P.J. / Silverman, D.N. / Christianson, D.W. #1:  Journal: J.Biol.Chem. / Year: 1994 Journal: J.Biol.Chem. / Year: 1994Title: Catalytic Properties of Mouse Carbonic Anhydrase V Authors: Heck, R.W. / Tanhauser, S.M. / Manda, R. / TU, C. / Laipis, P.J. / Silverman, D.N. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1dmy.cif.gz 1dmy.cif.gz | 109.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1dmy.ent.gz pdb1dmy.ent.gz | 84.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1dmy.json.gz 1dmy.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1dmy_validation.pdf.gz 1dmy_validation.pdf.gz | 400.7 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1dmy_full_validation.pdf.gz 1dmy_full_validation.pdf.gz | 409 KB | Display | |
| Data in XML |  1dmy_validation.xml.gz 1dmy_validation.xml.gz | 12.1 KB | Display | |
| Data in CIF |  1dmy_validation.cif.gz 1dmy_validation.cif.gz | 18.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dm/1dmy https://data.pdbj.org/pub/pdb/validation_reports/dm/1dmy ftp://data.pdbj.org/pub/pdb/validation_reports/dm/1dmy ftp://data.pdbj.org/pub/pdb/validation_reports/dm/1dmy | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO A 30 / 2: CIS PROLINE - PRO A 202 / 3: CIS PROLINE - PRO B 30 / 4: CIS PROLINE - PRO B 202 | ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.999912, 0.003781, -0.01269), Vector: Details | MTRIX THE TRANSFORMATIONS PRESENTED ON MTRIX RECORDS BELOW DESCRIBE NON-CRYSTALLOGRAPHIC RELATIONSHIPS AMONG THE VARIOUS DOMAINS IN THIS ENTRY. APPLYING THE APPROPRIATE MTRIX TRANSFORMATION TO THE RESIDUES LISTED FIRST WILL YIELD APPROXIMATE COORDINATES FOR THE RESIDUES LISTED SECOND. APPLIED TO TRANSFORMED TO MTRIX RESIDUES RESIDUES RMSD M1 A 25 .. A 261 B 25 .. B 261 0.445 | |
- Components
Components
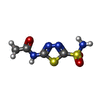
| #1: Protein | Mass: 28275.854 Da / Num. of mol.: 2 Mutation: TRUNCATION OF MITOCHONDRIAL LEADER SEQUENCE AND FIRST 21 RESIDUES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Description: TRUNCATED VERSION OF PROTEIN WITH 21 N TERMINAL RESIDUES REMOVED (SEE HECK ET AL., JBC, VOL 269 (1994) PP 24742-24746) Cell line: BL21 / Gene: MCA5C / Organ: LIVER / Organelle: MITOCHONDRIA / Plasmid: PET31 T7 / Gene (production host): MCA5C / Production host:  #2: Chemical | #3: Chemical | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.76 Å3/Da / Density % sol: 55.5 % |
|---|---|
| Crystal grow | pH: 7.2 / Details: pH 7.2 |
| Crystal grow | *PLUS pH: 7 / Method: unknown |
| Components of the solutions | *PLUS Common name: PEG8000 |
-Data collection
| Diffraction | Ambient temp details: ROOM |
|---|---|
| Diffraction source | Wavelength: 1.5418 |
| Detector | Type: R-AXIS IIC / Detector: IMAGE PLATE AREA DETECTOR / Date: Dec 19, 1994 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.45→19.9 Å / Num. obs: 21645 / % possible obs: 98 % / Observed criterion σ(I): 2 / Redundancy: 2 % / Rmerge(I) obs: 0.073 |
| Reflection | *PLUS Rmerge(I) obs: 0.073 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.45→8 Å / σ(F): 2 Details: CRYST1 TEXT TO EXPLAIN UNUSUAL UNIT-CELL DATA: TWO MOLECULES IN UNIT CELL RELATED BY NONCRYSTALLOGRAPHIC SYMMETRY SYMMETRY OPERATIONS FOR NON-STANDARD SETTING:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.45→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller













 PDBj
PDBj