+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1dj8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF E. COLI PERIPLASMIC PROTEIN HDEA | ||||||
 Components Components | PROTEIN HNS-DEPENDENT EXPRESSION A | ||||||
 Keywords Keywords | STRUCTURAL PROTEIN / ALPHA HELICAL | ||||||
| Function / homology |  Function and homology information Function and homology informationcellular stress response to acidic pH / cellular response to acidic pH / protein folding chaperone / unfolded protein binding / protein-folding chaperone binding / protein folding / outer membrane-bounded periplasmic space / protein homodimerization activity / identical protein binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2 Å SYNCHROTRON / Resolution: 2 Å | ||||||
 Authors Authors | Gajiwala, K.S. / Burley, S.K. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2000 Journal: J.Mol.Biol. / Year: 2000Title: HDEA, a periplasmic protein that supports acid resistance in pathogenic enteric bacteria. Authors: Gajiwala, K.S. / Burley, S.K. #1:  Journal: Nat.Struct.Biol. / Year: 1998 Journal: Nat.Struct.Biol. / Year: 1998Title: Crystal Structure of Escherichia coli HdeA Authors: Yang, F. / Gustafson, K.R. / Boyd, M.R. / Wlodawer, A. #2:  Journal: Electrophoresis / Year: 1997 Journal: Electrophoresis / Year: 1997Title: Comparing the Predicted and Observed Properties of Proteins Encoded in the Genome of Escherichia coli K-12 Authors: Link, A.J. / Robinson, K. / Church, G.H. #3:  Journal: Mol.Microbiol. / Year: 1996 Journal: Mol.Microbiol. / Year: 1996Title: Identification of Sigma S-Dependent Genes Associated with the Stationary-Phase Acid-Resistance Phenotype of Shigella flexneri Authors: Waterman, S.R. / Small, P.L. #4:  Journal: Biochem.Biophys.Res.Commun. / Year: 1996 Journal: Biochem.Biophys.Res.Commun. / Year: 1996Title: Evidence for GroES Acting as Transcriptional Regulator Authors: Legname, G. / Buono, P. / Fossati, G. / Monzini, N. / Mascagni, P. / Modena, D. / Marcucci, F. #5:  Journal: Mol.Microbiol. / Year: 1997 Journal: Mol.Microbiol. / Year: 1997Title: H-NS: a Modulator of Environmentally Regulated Gene Expression Authors: Atlung, T. / Ingmer, H. | ||||||
| History |
| ||||||
| Remark 99 | Author sent a new reflection file to supercede the initially released SF file in December, 1999. |
- Structure visualization
Structure visualization
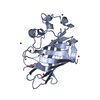
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1dj8.cif.gz 1dj8.cif.gz | 108.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1dj8.ent.gz pdb1dj8.ent.gz | 86.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1dj8.json.gz 1dj8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dj/1dj8 https://data.pdbj.org/pub/pdb/validation_reports/dj/1dj8 ftp://data.pdbj.org/pub/pdb/validation_reports/dj/1dj8 ftp://data.pdbj.org/pub/pdb/validation_reports/dj/1dj8 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 9752.882 Da / Num. of mol.: 6 / Source method: isolated from a natural source / Source: (natural)  #2: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.18 Å3/Da / Density % sol: 43.57 % | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion / pH: 4 Details: PEG1500, SODIUM ACETATE, pH 4, VAPOR DIFFUSION, temperature 298K | |||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, sitting drop | |||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CHESS CHESS  / Beamline: A1 / Wavelength: 0.908 / Beamline: A1 / Wavelength: 0.908 |
| Detector | Type: OTHER / Detector: CCD / Date: Dec 5, 1997 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.908 Å / Relative weight: 1 |
| Reflection | Resolution: 2→25 Å / Num. all: 33169 / Num. obs: 32222 / % possible obs: 97.2 % / Observed criterion σ(F): 3 / Observed criterion σ(I): 12 / Redundancy: 8 % / Biso Wilson estimate: 25.8 Å2 / Rmerge(I) obs: 0.062 / Net I/σ(I): 20.6 |
| Reflection shell | Resolution: 2→2.07 Å / Redundancy: 3 % / Rmerge(I) obs: 0.123 / % possible all: 97.7 |
| Reflection shell | *PLUS % possible obs: 97.7 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2→15 Å / σ(F): 3 / σ(I): 0 / Stereochemistry target values: ENGH & HUBER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→15 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: 'CNS' / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rwork: 0.246 |
 Movie
Movie Controller
Controller












 PDBj
PDBj
