+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 1cm4 | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Motions of calmodulin-four-conformer refinement | ||||||
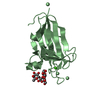
 要素 要素 |
| ||||||
 キーワード キーワード | CALCIUM-BINDING/TRANSFERASE / EF-HAND CALCIUM-BINDING PROTEIN / CALCIUM-BINDING-TRANSFERASE complex | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報regulation of synaptic vesicle docking / HSF1-dependent transactivation / RAF activation / regulation of store-operated calcium channel activity / Ion transport by P-type ATPases / peptidyl-threonine autophosphorylation / : / : / calcium- and calmodulin-dependent protein kinase complex / neurotransmitter receptor transport to plasma membrane ...regulation of synaptic vesicle docking / HSF1-dependent transactivation / RAF activation / regulation of store-operated calcium channel activity / Ion transport by P-type ATPases / peptidyl-threonine autophosphorylation / : / : / calcium- and calmodulin-dependent protein kinase complex / neurotransmitter receptor transport to plasma membrane / regulation of endocannabinoid signaling pathway / Interferon gamma signaling / regulation of response to tumor cell / positive regulation of autophagic cell death / DAPK1-calmodulin complex / Ca2+/calmodulin-dependent protein kinase / : / : / : / : / dendritic spine development / negative regulation of hydrolase activity / : / regulation of neurotransmitter secretion / Trafficking of AMPA receptors / establishment of protein localization to mitochondrial membrane / regulation of neuron migration / positive regulation of calcium ion transport / type 3 metabotropic glutamate receptor binding / Ca2+ pathway / establishment of protein localization to membrane / calcium/calmodulin-dependent protein kinase activity / GTPase activating protein binding / RAF/MAP kinase cascade / regulation of mitochondrial membrane permeability involved in apoptotic process / positive regulation of DNA binding / Ion homeostasis / negative regulation of high voltage-gated calcium channel activity / negative regulation of ryanodine-sensitive calcium-release channel activity / organelle localization by membrane tethering / dendrite morphogenesis / mitochondrion-endoplasmic reticulum membrane tethering / autophagosome membrane docking / negative regulation of calcium ion export across plasma membrane / regulation of cardiac muscle cell action potential / nitric-oxide synthase binding / regulation of neurotransmitter receptor localization to postsynaptic specialization membrane / regulation of synaptic vesicle exocytosis / adenylate cyclase binding / regulation of ryanodine-sensitive calcium-release channel activity / protein phosphatase activator activity / regulation of neuronal synaptic plasticity / catalytic complex / glutamate receptor binding / detection of calcium ion / regulation of synaptic vesicle endocytosis / Unblocking of NMDA receptors, glutamate binding and activation / regulation of cardiac muscle contraction / postsynaptic cytosol / activation of adenylate cyclase activity / positive regulation of cardiac muscle cell apoptotic process / regulation of protein localization to plasma membrane / cellular response to interferon-beta / phosphatidylinositol 3-kinase binding / calcium channel inhibitor activity / presynaptic cytosol / positive regulation of nitric-oxide synthase activity / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / enzyme regulator activity / titin binding / regulation of calcium-mediated signaling / voltage-gated potassium channel complex / potassium ion transmembrane transport / calcium channel complex / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / regulation of heart rate / response to amphetamine / adenylate cyclase activator activity / sarcomere / response to ischemia / nitric-oxide synthase regulator activity / regulation of cytokinesis / angiotensin-activated signaling pathway / spindle microtubule / calcium channel regulator activity / positive regulation of receptor signaling pathway via JAK-STAT / calcium-mediated signaling / G1/S transition of mitotic cell cycle / response to calcium ion / cellular response to type II interferon / G2/M transition of mitotic cell cycle / Schaffer collateral - CA1 synapse / spindle pole / disordered domain specific binding / calcium-dependent protein binding / calcium ion transport / kinase activity / myelin sheath / protein autophosphorylation 類似検索 - 分子機能 | ||||||
| 生物種 |  | ||||||
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  分子置換 / 解像度: 2 Å 分子置換 / 解像度: 2 Å | ||||||
 データ登録者 データ登録者 | Wall, M.E. / Phillips Jr., G.N. | ||||||
 引用 引用 |  ジャーナル: Structure / 年: 1997 ジャーナル: Structure / 年: 1997タイトル: Motions of calmodulin characterized using both Bragg and diffuse X-ray scattering. 著者: Wall, M.E. / Clarage, J.B. / Phillips Jr., G.N. #1:  ジャーナル: Science / 年: 1993 ジャーナル: Science / 年: 1993タイトル: Modulation of Calmodulin Plasticity in Molecular Recognition on the Basis of X-Ray Structures 著者: Meador, W.E. / Means, A.R. / Quiocho, F.A. #2:  ジャーナル: Science / 年: 1992 ジャーナル: Science / 年: 1992タイトル: Target Enzyme Recognition by Calmodulin: 2.4 A Structure of a Calmodulin-Peptide Complex 著者: Meador, W.E. / Means, A.R. / Quiocho, F.A. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  1cm4.cif.gz 1cm4.cif.gz | 119.8 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb1cm4.ent.gz pdb1cm4.ent.gz | 102.4 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  1cm4.json.gz 1cm4.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  1cm4_validation.pdf.gz 1cm4_validation.pdf.gz | 373.2 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  1cm4_full_validation.pdf.gz 1cm4_full_validation.pdf.gz | 381.9 KB | 表示 | |
| XML形式データ |  1cm4_validation.xml.gz 1cm4_validation.xml.gz | 7.5 KB | 表示 | |
| CIF形式データ |  1cm4_validation.cif.gz 1cm4_validation.cif.gz | 13.7 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/cm/1cm4 https://data.pdbj.org/pub/pdb/validation_reports/cm/1cm4 ftp://data.pdbj.org/pub/pdb/validation_reports/cm/1cm4 ftp://data.pdbj.org/pub/pdb/validation_reports/cm/1cm4 | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 単位格子 |
| ||||||||
| Components on special symmetry positions |
|
- 要素
要素
| #1: タンパク質 | 分子量: 16721.350 Da / 分子数: 1 / 由来タイプ: 天然 / 詳細: SIGMA LOT 54H9558 / 由来: (天然)  | ||
|---|---|---|---|
| #2: タンパク質・ペプチド | 分子量: 2886.528 Da / 分子数: 1 / Fragment: CALMODULIN BINDING DOMAIN, RESIDUES 290 - 314 / 由来タイプ: 合成 / 参照: UniProt: P11275, EC: 2.7.1.123 | ||
| #3: 化合物 | ChemComp-CA / #4: 水 | ChemComp-HOH / | |
-実験情報
-実験
| 実験 | 手法:  X線回折 / 使用した結晶の数: 1 X線回折 / 使用した結晶の数: 1 |
|---|
- 試料調製
試料調製
| 結晶 | マシュー密度: 2.23 Å3/Da / 溶媒含有率: 44.86 % | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 結晶化 | 手法: vapor diffusion - hanging drop - microseeding / pH: 5.2 詳細: DIFFRACTION-QUALITY CRYSTALS WERE MICROSEEDED IN HANGING DROPS OVER 100 MM SODIUM ACETATE AT PH 5.2, WITH 20% POLY-ETHYLENE GLYCOL 6000 (PEG 6000), 10 MM CALCIUM CHLORIDE AND 0.02% SODIUM ...詳細: DIFFRACTION-QUALITY CRYSTALS WERE MICROSEEDED IN HANGING DROPS OVER 100 MM SODIUM ACETATE AT PH 5.2, WITH 20% POLY-ETHYLENE GLYCOL 6000 (PEG 6000), 10 MM CALCIUM CHLORIDE AND 0.02% SODIUM AZIDE. STOCK SOLUTIONS OF 24 MG/ML BOVINE BRAIN CALMODULIN (SIGMA LOT 54H9558), 14 MG/ML CAMKII-ALPHA PEPTIDE, AND 30% PEG WERE MIXED INTO HANGING DROPS IN ABOUT A 4-2-1 RATIO., vapor diffusion - hanging drop - microseeding | ||||||||||||||||||||||||||||||||||||||||||||||||
| 結晶化 | *PLUS 手法: 蒸気拡散法, ハンギングドロップ法 / 詳細: used to seeding | ||||||||||||||||||||||||||||||||||||||||||||||||
| 溶液の組成 | *PLUS
|
-データ収集
| 回折 | 平均測定温度: 302 K |
|---|---|
| 放射光源 | 由来:  シンクロトロン / サイト: シンクロトロン / サイト:  CHESS CHESS  / ビームライン: F2 / 波長: 0.98 / ビームライン: F2 / 波長: 0.98 |
| 検出器 | タイプ: PRINCETON 2K / 検出器: CCD / 日付: 1996年5月1日 / 詳細: MIRRORS |
| 放射 | モノクロメーター: SINGLE-CRYSTAL / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray |
| 放射波長 | 波長: 0.98 Å / 相対比: 1 |
| 反射 | 解像度: 2→10 Å / Num. obs: 11522 / % possible obs: 92.1 % / Observed criterion σ(I): 2 / Biso Wilson estimate: 22.5 Å2 / Rsym value: 0.061 / Net I/σ(I): 9.2 |
| 反射 シェル | 解像度: 2→2.07 Å / Rsym value: 0.24 / % possible all: 82.8 |
- 解析
解析
| ソフトウェア |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 構造決定の手法:  分子置換 分子置換開始モデル: PDB ENTRY 1CM1 解像度: 2→10 Å / Rfactor Rfree error: 0.008 / Data cutoff high absF: 100000 / Data cutoff low absF: 0.1 / Isotropic thermal model: RESTRAINED / 交差検証法: THROUGHOUT / σ(F): 2 詳細: RESIDUES 74 - 83, WHICH ARE NOT PRESENT IN PDB ENTRY 1CDM, WERE ADDED FOR THIS REFINEMENT. AN INITIAL GUESS WAS OBTAINED FROM THE COORDINATES OF PDB ENTRY 1CDL. THESE RESIDUES DO NOT SHOW ...詳細: RESIDUES 74 - 83, WHICH ARE NOT PRESENT IN PDB ENTRY 1CDM, WERE ADDED FOR THIS REFINEMENT. AN INITIAL GUESS WAS OBTAINED FROM THE COORDINATES OF PDB ENTRY 1CDL. THESE RESIDUES DO NOT SHOW CONNECTED ELECTRON DENSITY AT A LEVEL OF 1SIGMA.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子変位パラメータ | Biso mean: 29.1 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: LAST / 解像度: 2→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS精密化 シェル | 解像度: 2→2.07 Å / Rfactor Rfree error: 0.023 / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ソフトウェア | *PLUS 名称:  X-PLOR / バージョン: 3.851 / 分類: refinement X-PLOR / バージョン: 3.851 / 分類: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 | *PLUS
|
 ムービー
ムービー コントローラー
コントローラー










 PDBj
PDBj








