[English] 日本語
 Yorodumi
Yorodumi- PDB-1cjt: COMPLEX OF GS-ALPHA WITH THE CATALYTIC DOMAINS OF MAMMALIAN ADENY... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1cjt | ||||||
|---|---|---|---|---|---|---|---|
| Title | COMPLEX OF GS-ALPHA WITH THE CATALYTIC DOMAINS OF MAMMALIAN ADENYLYL CYCLASE: COMPLEX WITH BETA-L-2',3'-DIDEOXYATP, MN, AND MG | ||||||
 Components Components |
| ||||||
 Keywords Keywords | LYASE/LYASE/SIGNALING PROTEIN / COMPLEX (LYASE-HYDROLASE) / HYDROLASE / SIGNAL TRANSDUCING PROTEIN / CYCLASE / EFFECTOR ENZYME / LYASE-LYASE-SIGNALING PROTEIN COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationAdenylate cyclase activating pathway / Hedgehog 'off' state / PKA activation / Adenylate cyclase inhibitory pathway / sensory perception of chemical stimulus / adenylate cyclase / regulation of insulin secretion involved in cellular response to glucose stimulus / cAMP biosynthetic process / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding ...Adenylate cyclase activating pathway / Hedgehog 'off' state / PKA activation / Adenylate cyclase inhibitory pathway / sensory perception of chemical stimulus / adenylate cyclase / regulation of insulin secretion involved in cellular response to glucose stimulus / cAMP biosynthetic process / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / adenylate cyclase activity / G alpha (z) signalling events / beta-2 adrenergic receptor binding / adenylate cyclase binding / D1 dopamine receptor binding / adenylate cyclase-activating adrenergic receptor signaling pathway / insulin-like growth factor receptor binding / ionotropic glutamate receptor binding / cellular response to forskolin / adenylate cyclase activator activity / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / adenylate cyclase-activating dopamine receptor signaling pathway / heterotrimeric G-protein complex / manganese ion binding / positive regulation of cytosolic calcium ion concentration / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / intracellular signal transduction / cilium / membrane raft / GTPase activity / dendrite / GTP binding / magnesium ion binding / protein-containing complex / ATP binding / metal ion binding / membrane / plasma membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |    | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.8 Å MOLECULAR REPLACEMENT / Resolution: 2.8 Å | ||||||
 Authors Authors | Tesmer, J.J.G. / Sprang, S.R. | ||||||
 Citation Citation |  Journal: Science / Year: 1999 Journal: Science / Year: 1999Title: Two-metal-Ion catalysis in adenylyl cyclase. Authors: Tesmer, J.J. / Sunahara, R.K. / Johnson, R.A. / Gosselin, G. / Gilman, A.G. / Sprang, S.R. #1:  Journal: Science / Year: 1997 Journal: Science / Year: 1997Title: Crystal Structure of the Catalytic Domains of Adenylyl Cyclase in a Complex with Gsalpha(Dot)Gtpgammas Authors: Tesmer, J.J. / Sunahara, R.K. / Gilman, A.G. / Sprang, S.R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1cjt.cif.gz 1cjt.cif.gz | 158.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1cjt.ent.gz pdb1cjt.ent.gz | 124.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1cjt.json.gz 1cjt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cj/1cjt https://data.pdbj.org/pub/pdb/validation_reports/cj/1cjt ftp://data.pdbj.org/pub/pdb/validation_reports/cj/1cjt ftp://data.pdbj.org/pub/pdb/validation_reports/cj/1cjt | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1cjkC  1cjuC  1cjvC  1azsS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
-ADENYLATE CYCLASE, TYPE ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 24495.361 Da / Num. of mol.: 1 / Fragment: C1A DOMAIN OF ADENYLYL CYCLASE / Mutation: V476M Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Protein | Mass: 23717.033 Da / Num. of mol.: 1 / Fragment: C2A DOMAIN OF ADENYLYL CYCLASE Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Protein , 1 types, 1 molecules C
| #3: Protein | Mass: 46656.438 Da / Num. of mol.: 1 / Fragment: TRYPSINIZED FRAGMENT / Mutation: N-TERMINAL HEXAHISTIDINE TAG Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 8 types, 74 molecules 


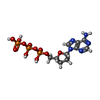











| #4: Chemical | | #5: Chemical | ChemComp-MN / | #6: Chemical | ChemComp-FOK / | #7: Chemical | ChemComp-DAD / | #8: Chemical | ChemComp-MES / | #9: Chemical | ChemComp-CL / | #10: Chemical | ChemComp-GSP / | #11: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3 Å3/Da / Density % sol: 58 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, hanging drop / pH: 5.6 Details: CRYSTALLIZED IN HANGING DROPS CONTAINING PROTEIN MIXED 1:1 WITH WELL SOLUTION OF 7.2-7.5% PEG 8000, 500MM NACL AND 100 MM PHOSPHATE BUFFER (PH 5.4-5.6), VAPOR DIFFUSION, HANGING DROP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion / Details: Tesmer, J.J., (1997) Science, 278, 1907. / PH range low: 5.6 / PH range high: 5.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CHESS CHESS  / Beamline: F2 / Wavelength: 0.923 / Beamline: F2 / Wavelength: 0.923 |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Aug 15, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.923 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→40 Å / Num. obs: 37320 / % possible obs: 98.8 % / Observed criterion σ(I): -3 / Redundancy: 3.4 % / Biso Wilson estimate: 33.7 Å2 / Rsym value: 0.106 / Net I/σ(I): 11.5 |
| Reflection shell | Resolution: 2.8→2.95 Å / Redundancy: 3.4 % / Mean I/σ(I) obs: 2.5 / Rsym value: 0.394 / % possible all: 100 |
| Reflection | *PLUS Rmerge(I) obs: 0.106 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1AZS Resolution: 2.8→15 Å / Rfactor Rfree error: 0.005 / Data cutoff high rms absF: 1744485.68 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 Details: A BULK SOLVENT CORRECTION WAS USED. REFLECTIONS WITH L>21 WERE OMITTED FROM REFINEMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 10 Å2 / ksol: 0.3107 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 41.3 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.8→15 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.8→2.9 Å / Rfactor Rfree error: 0.023 / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 0.5 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS σ(F): 0 / % reflection Rfree: 9.9 % / Rfactor obs: 0.206 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 41.3 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.349 / % reflection Rfree: 9.6 % / Rfactor Rwork: 0.307 |
 Movie
Movie Controller
Controller















 PDBj
PDBj
















