+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1c54 | ||||||
|---|---|---|---|---|---|---|---|
| Title | SOLUTION STRUCTURE OF RIBONUCLEASE SA | ||||||
 Components Components | RIBONUCLEASE SA | ||||||
 Keywords Keywords | HYDROLASE / ALPHA+BETA PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationribonuclease T1 / ribonuclease T1 activity / RNA endonuclease activity / lyase activity / RNA binding / extracellular region Similarity search - Function | ||||||
| Biological species |  Streptomyces aureofaciens (bacteria) Streptomyces aureofaciens (bacteria) | ||||||
| Method | SOLUTION NMR / molecular dynamics | ||||||
 Authors Authors | Laurents, D.V. / Canadillas-Perez, J.M. / Santoro, J. / Schell, D. / Pace, C.N. / Rico, M. / Bruix, M. | ||||||
 Citation Citation |  Journal: Proteins / Year: 2001 Journal: Proteins / Year: 2001Title: Solution structure and dynamics of ribonuclease Sa. Authors: Laurents, D. / Perez-Canadillas, J.M. / Santoro, J. / Rico, M. / Schell, D. / Pace, C.N. / Bruix, M. #1:  Journal: J.Biomol.NMR / Year: 1999 Journal: J.Biomol.NMR / Year: 1999Title: Sequential Assignment and Solution Secondary Structure of Doubly Labelled Ribonuclease Sa Authors: Laurents, D.V. / Canadillas-Perez, J.M. / Santoro, J. / Schell, D. / Pace, C.N. / Rico, M. / Bruix, M. #2:  Journal: Acta Crystallogr.,Sect.D / Year: 1996 Journal: Acta Crystallogr.,Sect.D / Year: 1996Title: Ribonucelase from Streptomyces Aureofaciens at Atomic Resolution Authors: Sevcik, J. / Dauter, Z. / Lamzin, V.S. / Wilson, K.S. | ||||||
| History |
|
- Structure visualization
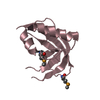
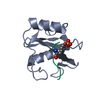
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1c54.cif.gz 1c54.cif.gz | 327.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1c54.ent.gz pdb1c54.ent.gz | 263.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1c54.json.gz 1c54.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1c54_validation.pdf.gz 1c54_validation.pdf.gz | 358.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1c54_full_validation.pdf.gz 1c54_full_validation.pdf.gz | 502.7 KB | Display | |
| Data in XML |  1c54_validation.xml.gz 1c54_validation.xml.gz | 34.2 KB | Display | |
| Data in CIF |  1c54_validation.cif.gz 1c54_validation.cif.gz | 57.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c5/1c54 https://data.pdbj.org/pub/pdb/validation_reports/c5/1c54 ftp://data.pdbj.org/pub/pdb/validation_reports/c5/1c54 ftp://data.pdbj.org/pub/pdb/validation_reports/c5/1c54 | HTTPS FTP |
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 10582.492 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Streptomyces aureofaciens (bacteria) / Plasmid: PEH100 / Production host: Streptomyces aureofaciens (bacteria) / Plasmid: PEH100 / Production host:  |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||
| NMR details | Text: THIS STRUCTURE WAS DETERMINED WITH A 80MS MIXING TIME, AND STANDARD 2D HOMONUCLEAR TECHNIQUES. |
- Sample preparation
Sample preparation
| Details |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample conditions |
| |||||||||||||||||||||||||
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer | Type: Bruker AMX / Manufacturer: Bruker / Model: AMX / Field strength: 600 MHz |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: molecular dynamics / Software ordinal: 1 Details: THE STRUCTURES ARE BASED ON A TOTAL OF 1924 UPPER DISTANCE RESTRAINTS, DERIVED FROM 2276 UNAMBIGUOUS NOES, AND 3 LOWER DISTANCE RESTRAINTS BASED ON THE PROTEINS C7-C96 DISULFIDE BOND. | ||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: LOWEST ENERGY AND REPRESENTATIVE OF DIFFERENT CONFORMERS Conformers calculated total number: 40 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller











 PDBj
PDBj