+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1b98 | ||||||
|---|---|---|---|---|---|---|---|
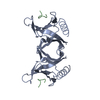
| Title | NEUROTROPHIN 4 (HOMODIMER) | ||||||
 Components Components | PROTEIN (NEUROTROPHIN-4) | ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / TARGET-DERIVED SURVIVAL FACTOR / NEUROTROPHIN 4 / NEUROTROPHIN 5 / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationtaste bud development / sensory organ boundary specification / ganglion mother cell fate determination / NTF4 activates NTRK2 (TRKB) signaling / Activated NTRK2 signals through PLCG1 / nerve growth factor receptor binding / ameloblast differentiation / mechanoreceptor differentiation / nerve growth factor signaling pathway / nerve development ...taste bud development / sensory organ boundary specification / ganglion mother cell fate determination / NTF4 activates NTRK2 (TRKB) signaling / Activated NTRK2 signals through PLCG1 / nerve growth factor receptor binding / ameloblast differentiation / mechanoreceptor differentiation / nerve growth factor signaling pathway / nerve development / Activated NTRK2 signals through PI3K / innervation / Activated NTRK2 signals through RAS / epidermis development / long-term memory / Activated NTRK2 signals through FRS2 and FRS3 / neuron projection morphogenesis / cell surface receptor protein tyrosine kinase signaling pathway / adult locomotory behavior / growth factor activity / modulation of chemical synaptic transmission / Constitutive Signaling by Aberrant PI3K in Cancer / synaptic vesicle / PIP3 activates AKT signaling / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / negative regulation of neuron apoptotic process / axon / dendrite / extracellular space / extracellular region Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.75 Å MOLECULAR REPLACEMENT / Resolution: 2.75 Å | ||||||
 Authors Authors | Robinson, R.C. / Radziejewski, C. / Stuart, D.I. / Jones, E.Y. / Choe, S. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 1999 Journal: Protein Sci. / Year: 1999Title: The structures of the neurotrophin 4 homodimer and the brain-derived neurotrophic factor/neurotrophin 4 heterodimer reveal a common Trk-binding site. Authors: Robinson, R.C. / Radziejewski, C. / Spraggon, G. / Greenwald, J. / Kostura, M.R. / Burtnick, L.D. / Stuart, D.I. / Choe, S. / Jones, E.Y. #1:  Journal: Biochemistry / Year: 1995 Journal: Biochemistry / Year: 1995Title: Structure of the Brain-Derived Neurotrophic Factor/Neurotrophin 3 Heterodimer Authors: Robinson, R.C. / Radziejewski, C. / Stuart, D.I. / Jones, E.Y. #2:  Journal: Biochemistry / Year: 1993 Journal: Biochemistry / Year: 1993Title: Heterodimers of the Neurotrophic Factors: Formation, Isolation, and Differential Stability Authors: Radziejewski, C. / Robinson, R.C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1b98.cif.gz 1b98.cif.gz | 55.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1b98.ent.gz pdb1b98.ent.gz | 39.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1b98.json.gz 1b98.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b9/1b98 https://data.pdbj.org/pub/pdb/validation_reports/b9/1b98 ftp://data.pdbj.org/pub/pdb/validation_reports/b9/1b98 ftp://data.pdbj.org/pub/pdb/validation_reports/b9/1b98 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 13944.647 Da / Num. of mol.: 2 / Fragment: precursor residues 81-210 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Description: SUPPLIED BY REGENERON PHARMACUTICALS / Production host: Homo sapiens (human) / Description: SUPPLIED BY REGENERON PHARMACUTICALS / Production host:  #2: Chemical | ChemComp-CL / | #3: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.99 Å3/Da / Density % sol: 38.3 % | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 6.5 / Details: PEG 8000, PIPES, pH 6.50 | ||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 15 ℃ / pH: 8 / Method: vapor diffusion | ||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL1-5 / Wavelength: 0.97 / Beamline: BL1-5 / Wavelength: 0.97 |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97 Å / Relative weight: 1 |
| Reflection | Resolution: 2.75→20 Å / Num. obs: 7858 / % possible obs: 93 % / Redundancy: 2.5 % / Rmerge(I) obs: 0.031 |
| Reflection | *PLUS Highest resolution: 2.35 Å |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: NT4 Resolution: 2.75→20 Å / Cross valid method: THROUGHOUT / σ(F): 0
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 46.3 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.75→20 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: REFMAC / Classification: refinement | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.35 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller














 PDBj
PDBj






