+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1a1f | ||||||
|---|---|---|---|---|---|---|---|
| Title | DSNR (ZIF268 VARIANT) ZINC FINGER-DNA COMPLEX (GACC SITE) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION/DNA / COMPLEX (ZINC FINGER-DNA) / ZINC FINGER / DNA-BINDING PROTEIN / TRANSCRIPTION-DNA COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of protein sumoylation / glomerular mesangial cell proliferation / positive regulation of glomerular metanephric mesangial cell proliferation / cellular response to interleukin-8 / regulation of progesterone biosynthetic process / cellular response to heparin / cellular response to mycophenolic acid / circadian temperature homeostasis / positive regulation of post-translational protein modification / positive regulation of hormone biosynthetic process ...regulation of protein sumoylation / glomerular mesangial cell proliferation / positive regulation of glomerular metanephric mesangial cell proliferation / cellular response to interleukin-8 / regulation of progesterone biosynthetic process / cellular response to heparin / cellular response to mycophenolic acid / circadian temperature homeostasis / positive regulation of post-translational protein modification / positive regulation of hormone biosynthetic process / double-stranded methylated DNA binding / hemi-methylated DNA-binding / positive regulation of gene expression via chromosomal CpG island demethylation / interleukin-1-mediated signaling pathway / histone acetyltransferase binding / locomotor rhythm / T cell differentiation / skeletal muscle cell differentiation / BMP signaling pathway / response to glucose / estrous cycle / positive regulation of chemokine production / regulation of neuron apoptotic process / response to ischemia / positive regulation of interleukin-1 beta production / RNA polymerase II transcription regulatory region sequence-specific DNA binding / circadian regulation of gene expression / promoter-specific chromatin binding / negative regulation of canonical Wnt signaling pathway / cellular response to gamma radiation / response to insulin / positive regulation of miRNA transcription / DNA-binding transcription activator activity, RNA polymerase II-specific / regulation of apoptotic process / sequence-specific DNA binding / response to hypoxia / transcription cis-regulatory region binding / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / chromatin / positive regulation of DNA-templated transcription / enzyme binding / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / DNA binding / zinc ion binding / nucleoplasm / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / ISOMORPHOUS MOLECULAR REPLACEMENT / Resolution: 2.1 Å X-RAY DIFFRACTION / ISOMORPHOUS MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Elrod-Erickson, M. / Benson, T.E. / Pabo, C.O. | ||||||
 Citation Citation |  Journal: Structure / Year: 1998 Journal: Structure / Year: 1998Title: High-resolution structures of variant Zif268-DNA complexes: implications for understanding zinc finger-DNA recognition. Authors: Elrod-Erickson, M. / Benson, T.E. / Pabo, C.O. #1:  Journal: Structure / Year: 1996 Journal: Structure / Year: 1996Title: Zif268 Protein-DNA Complex Refined at 1.6 A: A Model System for Understanding Zinc Finger-DNA Interactions Authors: Elrod-Erickson, M. / Rould, M.A. / Nekludova, L. / Pabo, C.O. #2:  Journal: Science / Year: 1994 Journal: Science / Year: 1994Title: Zinc Finger Phage: Affinity Selection of Fingers with New DNA-Binding Specificities Authors: Rebar, E.J. / Pabo, C.O. #3:  Journal: Science / Year: 1991 Journal: Science / Year: 1991Title: Zinc Finger-DNA Recognition: Crystal Structure of a Zif268-DNA Complex at 2.1 A Authors: Pavletich, N.P. / Pabo, C.O. | ||||||
| History |
|
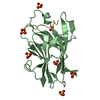
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1a1f.cif.gz 1a1f.cif.gz | 42 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1a1f.ent.gz pdb1a1f.ent.gz | 30.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1a1f.json.gz 1a1f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1a1f_validation.pdf.gz 1a1f_validation.pdf.gz | 375.4 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1a1f_full_validation.pdf.gz 1a1f_full_validation.pdf.gz | 377 KB | Display | |
| Data in XML |  1a1f_validation.xml.gz 1a1f_validation.xml.gz | 3.6 KB | Display | |
| Data in CIF |  1a1f_validation.cif.gz 1a1f_validation.cif.gz | 5.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/a1/1a1f https://data.pdbj.org/pub/pdb/validation_reports/a1/1a1f ftp://data.pdbj.org/pub/pdb/validation_reports/a1/1a1f ftp://data.pdbj.org/pub/pdb/validation_reports/a1/1a1f | HTTPS FTP |
-Related structure data
| Related structure data |  1a1gC  1a1hC  1a1iC  1a1jC  1a1kC  1a1lC  1aayS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
| #1: DNA chain | Mass: 3399.223 Da / Num. of mol.: 1 / Source method: obtained synthetically | ||
|---|---|---|---|
| #2: DNA chain | Mass: 3310.161 Da / Num. of mol.: 1 / Source method: obtained synthetically | ||
| #3: Protein | Mass: 10768.371 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   | ||
| #4: Chemical | | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.17 Å3/Da / Density % sol: 43.29 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, hanging drop / pH: 7.5 Details: 20% PEG 400, 200MM MGCL2, 100 MM HEPES PH 7.5, VAPOR DIFFUSION, HANGING DROP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 6.2 / Details: macroseeding | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 130 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS IIC / Detector: IMAGE PLATE / Date: Nov 1, 1995 / Details: YALE MIRRORS |
| Radiation | Monochromator: YALE MIRRORS / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→20 Å / Num. obs: 8185 / % possible obs: 87 % / Observed criterion σ(I): -2 / Redundancy: 2.4 % / Rsym value: 0.064 / Net I/σ(I): 22.6 |
| Reflection shell | Resolution: 2.1→2.17 Å / Redundancy: 1.8 % / Mean I/σ(I) obs: 10.9 / Rsym value: 0.093 / % possible all: 76 |
| Reflection | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 20 Å / % possible obs: 87 % / Redundancy: 2.4 % / Num. measured all: 19390 / Rmerge(I) obs: 0.064 |
| Reflection shell | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 2.17 Å / % possible obs: 76 % / Redundancy: 1.8 % / Mean I/σ(I) obs: 10.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: ISOMORPHOUS MOLECULAR REPLACEMENT Starting model: PDB ENTRY 1AAY, WITHOUT WATERS AND WITHOUT SIDE CHAINS FOR RESIDUES 18 - 24 Resolution: 2.1→20 Å / Rfactor Rfree error: 0.009 / Data cutoff high absF: 100000 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED INDIVIDUAL / Cross valid method: THROUGHOUT / σ(F): 0 / Details: RMS DEVIATIONS FROM IDEAL VALUES (DNA)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 36.4 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.1→2.2 Å / Rfactor Rfree error: 0.044 / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.8 / Classification: refinement X-PLOR / Version: 3.8 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 20 Å / Num. reflection obs: 8173 / σ(F): 0 / % reflection Rfree: 10.9 % / Rfactor obs: 0.23 / Rfactor Rfree: 0.27 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor obs: 0.359 |
 Movie
Movie Controller
Controller












 PDBj
PDBj










































