[English] 日本語
 Yorodumi
Yorodumi- EMDB-9382: A high-resolution cryo-electron microscopy structure of a calcito... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9382 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | A high-resolution cryo-electron microscopy structure of a calcitonin receptor-heterotrimeric Gs protein complex | |||||||||||||||
 Map data Map data | Calcitonin receptor-heterotrimeric Gs protein complex, Sharpened map (B -80) | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | GPCR / transmembrane / receptor / calcitonin / MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcalcitonin receptor binding / calcitonin binding / calcitonin family receptor activity / amylin receptor complex 1 / amylin receptor complex 2 / calcitonin family receptor signaling pathway / amylin receptor complex 3 / amylin receptor activity / calcitonin receptor activity / calcitonin gene-related peptide receptor signaling pathway ...calcitonin receptor binding / calcitonin binding / calcitonin family receptor activity / amylin receptor complex 1 / amylin receptor complex 2 / calcitonin family receptor signaling pathway / amylin receptor complex 3 / amylin receptor activity / calcitonin receptor activity / calcitonin gene-related peptide receptor signaling pathway / calcitonin gene-related peptide receptor activity / amylin receptor 3 signaling pathway / amylin receptor 2 signaling pathway / amylin receptor 1 signaling pathway / amylin receptor signaling pathway / Calcitonin-like ligand receptors / negative regulation of ossification / response to amyloid-beta / positive regulation of cAMP/PKA signal transduction / PKA activation in glucagon signalling / developmental growth / hair follicle placode formation / intracellular transport / D1 dopamine receptor binding / vascular endothelial cell response to laminar fluid shear stress / activation of adenylate cyclase activity / renal water homeostasis / Hedgehog 'off' state / adenylate cyclase-activating adrenergic receptor signaling pathway / regulation of mRNA stability / regulation of insulin secretion / cellular response to glucagon stimulus / positive regulation of calcium-mediated signaling / acrosomal vesicle / osteoclast differentiation / response to glucocorticoid / ossification / adenylate cyclase activator activity / trans-Golgi network membrane / negative regulation of inflammatory response to antigenic stimulus / hormone activity / bone development / platelet aggregation / cognition / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / sensory perception of smell / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / positive regulation of cold-induced thermogenesis / amyloid-beta binding / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / positive regulation of cytosolic calcium ion concentration / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Ras protein signal transduction Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||||||||
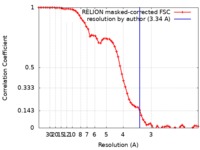
| Method | single particle reconstruction / cryo EM / Resolution: 3.34 Å | |||||||||||||||
 Authors Authors | dal Maso E / Glukhova A | |||||||||||||||
| Funding support |  Australia, 4 items Australia, 4 items
| |||||||||||||||
 Citation Citation |  Journal: ACS Pharmacol Transl Sci / Year: 2019 Journal: ACS Pharmacol Transl Sci / Year: 2019Title: The Molecular Control of Calcitonin Receptor Signaling. Authors: Emma Dal Maso / Alisa Glukhova / Yue Zhu / Javier Garcia-Nafria / Christopher G Tate / Silvia Atanasio / Christopher A Reynolds / Erney Ramírez-Aportela / Jose-Maria Carazo / Caroline A ...Authors: Emma Dal Maso / Alisa Glukhova / Yue Zhu / Javier Garcia-Nafria / Christopher G Tate / Silvia Atanasio / Christopher A Reynolds / Erney Ramírez-Aportela / Jose-Maria Carazo / Caroline A Hick / Sebastian G B Furness / Debbie L Hay / Yi-Lynn Liang / Laurence J Miller / Arthur Christopoulos / Ming-Wei Wang / Denise Wootten / Patrick M Sexton /       Abstract: The calcitonin receptor (CTR) is a class B G protein-coupled receptor (GPCR) that responds to the peptide hormone calcitonin (CT). CTs are clinically approved for the treatment of bone diseases. We ...The calcitonin receptor (CTR) is a class B G protein-coupled receptor (GPCR) that responds to the peptide hormone calcitonin (CT). CTs are clinically approved for the treatment of bone diseases. We previously reported a 4.1 Å structure of the activated CTR bound to salmon CT (sCT) and heterotrimeric Gs protein by cryo-electron microscopy (Liang, Y.-L., . Phase-plate cryo- EM structure of a class B GPCR-G protein complex. , , 118-123). In the current study, we have reprocessed the electron micrographs to yield a 3.3 Å map of the complex. This has allowed us to model extracellular loops (ECLs) 2 and 3, and the peptide N-terminus that previously could not be resolved. We have also performed alanine scanning mutagenesis of ECL1 and the upper segment of transmembrane helix 1 (TM1) and its extension into the receptor extracellular domain (TM1 stalk), with effects on peptide binding and function assessed by cAMP accumulation and ERK1/2 phosphorylation. These data were combined with previously published alanine scanning mutagenesis of ECL2 and ECL3 and the new structural information to provide a comprehensive 3D map of the molecular surface of the CTR that controls binding and signaling of distinct CT and related peptides. The work highlights distinctions in how different, related, class B receptors may be activated. The new mutational data on the TM1 stalk and ECL1 have also provided critical insights into the divergent control of cAMP versus pERK signaling and, collectively with previous mutagenesis data, offer evidence that the conformations linked to these different signaling pathways are, in many ways, mutually exclusive. This study furthers our understanding of the complex nature of signaling elicited by GPCRs and, in particular, that of the therapeutically important class B subfamily. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9382.map.gz emd_9382.map.gz | 2.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9382-v30.xml emd-9382-v30.xml emd-9382.xml emd-9382.xml | 28.3 KB 28.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9382_fsc.xml emd_9382_fsc.xml | 6.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_9382.png emd_9382.png | 106.7 KB | ||
| Masks |  emd_9382_msk_1.map emd_9382_msk_1.map | 22.2 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-9382.cif.gz emd-9382.cif.gz | 7.6 KB | ||
| Others |  emd_9382_additional_1.map.gz emd_9382_additional_1.map.gz emd_9382_additional_2.map.gz emd_9382_additional_2.map.gz emd_9382_half_map_1.map.gz emd_9382_half_map_1.map.gz emd_9382_half_map_2.map.gz emd_9382_half_map_2.map.gz | 20.9 MB 16.9 MB 17 MB 17 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9382 http://ftp.pdbj.org/pub/emdb/structures/EMD-9382 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9382 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9382 | HTTPS FTP |
-Related structure data
| Related structure data |  6niyMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9382.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9382.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Calcitonin receptor-heterotrimeric Gs protein complex, Sharpened map (B -80) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_9382_msk_1.map emd_9382_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Calcitonin receptor-heterotrimeric Gs protein complex, LocalDeblur sharpened map...
| File | emd_9382_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Calcitonin receptor-heterotrimeric Gs protein complex, LocalDeblur sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Calcitonin receptor-heterotrimeric Gs protein complex, unsharpened map...
| File | emd_9382_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Calcitonin receptor-heterotrimeric Gs protein complex, unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Calcitonin receptor-heterotrimeric Gs protein complex, final half map...
| File | emd_9382_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Calcitonin receptor-heterotrimeric Gs protein complex, final half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Calcitonin receptor-heterotrimeric Gs protein complex, final half map...
| File | emd_9382_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Calcitonin receptor-heterotrimeric Gs protein complex, final half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of a full-length, active-state calcitonin receptor with p...
| Entire | Name: Complex of a full-length, active-state calcitonin receptor with peptide ligand, heterotrimeric Gs protein and nanobody 35 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of a full-length, active-state calcitonin receptor with p...
| Supramolecule | Name: Complex of a full-length, active-state calcitonin receptor with peptide ligand, heterotrimeric Gs protein and nanobody 35 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 150 KDa |
-Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.72552 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKSTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE YQLIDCAQYF LDKIDVIKQA DYVPSDQDLL RCRVLTSGIF ETKFQVDKVN FHMFDVGGQR DERRKWIQCF ND VTAIIFV VASSSYNMVI REDNQTNRLQ EALNLFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK IEDYFPEFAR YTT PEDATP EPGEDPRVTR AKYFIRDEFL RISTASGDGR HYCYPHFTCA VDTENIRRVF NDCRDIIQRM HLRQYELL UniProtKB: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #2: Nanobody35
| Macromolecule | Name: Nanobody35 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.140742 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSSH HHHHHEPEA |
-Macromolecule #3: Calcitonin receptor
| Macromolecule | Name: Calcitonin receptor / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55.387316 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MRFTFTSRCL ALFLLLNHPT PILPAFSNQT YPTIEPKPFL YVVGRKKMMD AQYKCYDRMQ QLPAYQGEGP YCNRTWDGWL CWDDTPAGV LSYQFCPDYF PDFDPSEKVT KYCDEKGVWF KHPENNRTWS NYTMCNAFTP EKLKNAYVLY YLAIVGHSLS I FTLVISLG ...String: MRFTFTSRCL ALFLLLNHPT PILPAFSNQT YPTIEPKPFL YVVGRKKMMD AQYKCYDRMQ QLPAYQGEGP YCNRTWDGWL CWDDTPAGV LSYQFCPDYF PDFDPSEKVT KYCDEKGVWF KHPENNRTWS NYTMCNAFTP EKLKNAYVLY YLAIVGHSLS I FTLVISLG IFVFFRSLGC QRVTLHKNMF LTYILNSMII IIHLVEVVPN GELVRRDPVS CKILHFFHQY MMACNYFWML CE GIYLHTL IVVAVFTEKQ RLRWYYLLGW GFPLVPTTIH AITRAVYFND NCWLSVETHL LYIIHGPVMA ALVVNFFFLL NIV RVLVTK MRETHEAESH MYLKAVKATM ILVPLLGIQF VVFPWRPSNK MLGKIYDYVM HSLIHFQGFF VATIYCFCNN EVQT TVKRQ WAQFKIQWNQ RWGRRPSNRS ARAAAAAAEA GDIPIYICHQ EPRNEPANNQ GEESAEIIPL NIIEQESSA UniProtKB: Calcitonin receptor |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.41693 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #6: Calcitonin
| Macromolecule | Name: Calcitonin / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 3.438886 KDa |
| Sequence | String: CSNLSTCVLG KLSQELHKLQ TYPRTNTGSG TP UniProtKB: Calcitonin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: OTHER |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 47170 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)