+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-8376 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of rabbit RyR1 (Caffeine/ATP/Ca2+ dataset, class 1) | |||||||||
 マップデータ マップデータ | Rabbit RyR1-Cs2 complex, Ca2+/ATP/caffine dataset, class 1. Aligned to transmembrane region of EMD-8342 to facilitate class comparison. Used for model fitting. | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | RyR / Ca2+ / EC coupling / gating / TRANSPORT PROTEIN-ISOMERASE complex | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報ATP-gated ion channel activity / positive regulation of sequestering of calcium ion / negative regulation of calcium-mediated signaling / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / insulin secretion involved in cellular response to glucose stimulus / ryanodine-sensitive calcium-release channel activity / terminal cisterna / ryanodine receptor complex ...ATP-gated ion channel activity / positive regulation of sequestering of calcium ion / negative regulation of calcium-mediated signaling / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / insulin secretion involved in cellular response to glucose stimulus / ryanodine-sensitive calcium-release channel activity / terminal cisterna / ryanodine receptor complex / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / response to redox state / ossification involved in bone maturation / 'de novo' protein folding / negative regulation of heart rate / cellular response to caffeine / skin development / FK506 binding / organelle membrane / intracellularly gated calcium channel activity / smooth endoplasmic reticulum / outflow tract morphogenesis / smooth muscle contraction / toxic substance binding / T cell proliferation / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / striated muscle contraction / voltage-gated calcium channel activity / calcium channel inhibitor activity / skeletal muscle fiber development / Ion homeostasis / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / release of sequestered calcium ion into cytosol / calcium channel complex / sarcoplasmic reticulum membrane / muscle contraction / cellular response to calcium ion / protein maturation / sarcoplasmic reticulum / calcium channel regulator activity / peptidylprolyl isomerase / peptidyl-prolyl cis-trans isomerase activity / calcium-mediated signaling / sarcolemma / calcium ion transmembrane transport / calcium channel activity / Stimuli-sensing channels / Z disc / intracellular calcium ion homeostasis / disordered domain specific binding / positive regulation of cytosolic calcium ion concentration / protein refolding / protein homotetramerization / transmembrane transporter binding / calmodulin binding / signaling receptor binding / calcium ion binding / ATP binding / identical protein binding / membrane / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /  | |||||||||
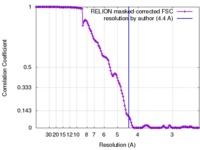
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 4.4 Å | |||||||||
 データ登録者 データ登録者 | Clarke OB / des Georges A | |||||||||
 引用 引用 |  ジャーナル: Cell / 年: 2016 ジャーナル: Cell / 年: 2016タイトル: Structural Basis for Gating and Activation of RyR1. 著者: Amédée des Georges / Oliver B Clarke / Ran Zalk / Qi Yuan / Kendall J Condon / Robert A Grassucci / Wayne A Hendrickson / Andrew R Marks / Joachim Frank /  要旨: The type-1 ryanodine receptor (RyR1) is an intracellular calcium (Ca(2+)) release channel required for skeletal muscle contraction. Here, we present cryo-EM reconstructions of RyR1 in multiple ...The type-1 ryanodine receptor (RyR1) is an intracellular calcium (Ca(2+)) release channel required for skeletal muscle contraction. Here, we present cryo-EM reconstructions of RyR1 in multiple functional states revealing the structural basis of channel gating and ligand-dependent activation. Binding sites for the channel activators Ca(2+), ATP, and caffeine were identified at interdomain interfaces of the C-terminal domain. Either ATP or Ca(2+) alone induces conformational changes in the cytoplasmic assembly ("priming"), without pore dilation. In contrast, in the presence of all three activating ligands, high-resolution reconstructions of open and closed states of RyR1 were obtained from the same sample, enabling analyses of conformational changes associated with gating. Gating involves global conformational changes in the cytosolic assembly accompanied by local changes in the transmembrane domain, which include bending of the S6 transmembrane segment and consequent pore dilation, displacement, and deformation of the S4-S5 linker and conformational changes in the pseudo-voltage-sensor domain. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_8376.map.gz emd_8376.map.gz | 224.8 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-8376-v30.xml emd-8376-v30.xml emd-8376.xml emd-8376.xml | 32.6 KB 32.6 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_8376_fsc.xml emd_8376_fsc.xml | 13.8 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_8376.png emd_8376.png | 106.9 KB | ||
| Filedesc metadata |  emd-8376.cif.gz emd-8376.cif.gz | 8.6 KB | ||
| その他 |  emd_8376_additional.map.gz emd_8376_additional.map.gz emd_8376_half_map_1.map.gz emd_8376_half_map_1.map.gz emd_8376_half_map_2.map.gz emd_8376_half_map_2.map.gz | 227.9 MB 188.8 MB 188.2 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8376 http://ftp.pdbj.org/pub/emdb/structures/EMD-8376 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8376 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8376 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_8376_validation.pdf.gz emd_8376_validation.pdf.gz | 971 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_8376_full_validation.pdf.gz emd_8376_full_validation.pdf.gz | 970.6 KB | 表示 | |
| XML形式データ |  emd_8376_validation.xml.gz emd_8376_validation.xml.gz | 20.8 KB | 表示 | |
| CIF形式データ |  emd_8376_validation.cif.gz emd_8376_validation.cif.gz | 27.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8376 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8376 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8376 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8376 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  5t9vMC  8342C  8372C  8373C  8374C  8375C  8377C  8378C  8379C  8380C  8381C  8382C  8383C  8384C  8385C  8386C  8387C  8388C  8389C  8390C  8391C  8392C  8393C  8394C  8395C  5t15C  5t9mC  5t9nC  5t9rC  5t9sC  5ta3C  5talC  5tamC  5tanC  5tapC  5taqC  5tasC  5tatC  5tauC  5tavC  5tawC  5taxC  5tayC  5tazC  5tb0C  5tb1C  5tb2C  5tb3C  5tb4C C: 同じ文献を引用 ( M: このマップから作成された原子モデル |
|---|---|
| 類似構造データ |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_8376.map.gz / 形式: CCP4 / 大きさ: 244.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_8376.map.gz / 形式: CCP4 / 大きさ: 244.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Rabbit RyR1-Cs2 complex, Ca2+/ATP/caffine dataset, class 1. Aligned to transmembrane region of EMD-8342 to facilitate class comparison. Used for model fitting. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.255 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
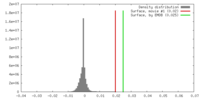
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-追加マップ: Main map (untransformed)
| ファイル | emd_8376_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Main map (untransformed) | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
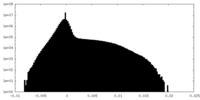
| 密度ヒストグラム |
-ハーフマップ: Half map 2
| ファイル | emd_8376_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Half map 2 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
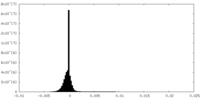
| 密度ヒストグラム |
-ハーフマップ: Half map 1
| ファイル | emd_8376_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Half map 1 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
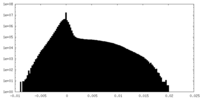
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : RyR1-Cs2 complex
| 全体 | 名称: RyR1-Cs2 complex |
|---|---|
| 要素 |
|
-超分子 #1: RyR1-Cs2 complex
| 超分子 | 名称: RyR1-Cs2 complex / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#2 |
|---|
-分子 #1: Peptidyl-prolyl cis-trans isomerase FKBP1B
| 分子 | 名称: Peptidyl-prolyl cis-trans isomerase FKBP1B / タイプ: protein_or_peptide / ID: 1 / コピー数: 4 / 光学異性体: LEVO / EC番号: peptidylprolyl isomerase |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 11.798501 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGVEIETISP GDGRTFPKKG QTCVVHYTGM LQNGKKFDSS RDRNKPFKFR IGKQEVIKGF EEGAAQMSLG QRAKLTCTPD VAYGATGHP GVIPPNATLI FDVELLNLE UniProtKB: Peptidyl-prolyl cis-trans isomerase FKBP1B |
-分子 #2: Ryanodine receptor 1
| 分子 | 名称: Ryanodine receptor 1 / タイプ: protein_or_peptide / ID: 2 / コピー数: 4 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 475.107719 KDa |
| 配列 | 文字列: QFLRTDDEVV LQCSATVLKE QLKLCLAAEG FGNRLCFLEP TSNAQNVPPD LAICCFTLEQ SLSVRALQEM LANTVEAGVE SSQGGGHRT LLYGHAILLR HAHSRMYLSC LTTSRSMTDK LAFDVGLQED ATGEACWWTM HPASKQRSEG EKVRVGDDLI L VSVSSERY ...文字列: QFLRTDDEVV LQCSATVLKE QLKLCLAAEG FGNRLCFLEP TSNAQNVPPD LAICCFTLEQ SLSVRALQEM LANTVEAGVE SSQGGGHRT LLYGHAILLR HAHSRMYLSC LTTSRSMTDK LAFDVGLQED ATGEACWWTM HPASKQRSEG EKVRVGDDLI L VSVSSERY LHLSTASGEL QVDASFMQTL WNMNPICSCC EEGYVTGGHV LRLFHGHMDE CLTISAADSD DQRRLVYYEG GA VCTHARS LWRLEPLRIS WSGSHLRWGQ PLRIRHVTTG RYLALTEDQG LVVVDACKAH TKATSFCFRV SKEKLDTAPK RDV EGMGPP EIKYGESLCF VQHVASGLWL TYAAPDPKAL RLGVLKKKAI LHQEGHMDDA LFLTRCQQEE SQAARMIHST AGLY NQFIK GLDSFSGKPR GSGPPAGPAL PIEAVILSLQ DLIGYFEPPS EELQHEEKQS KLRSLRNRQS LFQEEGMLSL VLNCI DRLN VYTTAAHFAE YAGEEAAESW KEIVNLLYEL LASLIRGNRA NCALFSTNLD WVVSKLDRLE ASSGILEVLY CVLIES PEV LNIIQENHIK SIISLLDKHG RNHKVLDVLC SLCVCNGVAV RSNQDLITEN LLPGRELLLQ TNLINYVTSI RPNIFVG RA EGSTQYGKWY FEVMVDEVVP FLTAQATHLR VGWALTEGYS PYPGGGEGWG GNGVGDDLYS YGFDGLHLWT GHVARPVT S PGQHLLAPED VVSCCLDLSV PSISFRINGC PVQGVFEAFN LDGLFFPVVS FSAGVKVRFL LGGRHGEFKF LPPPGYAPC HEAVLPRERL RLEPIKEYRR EGPRGPHLVG PSRCLSHTDF VPCPVDTVQI VLPPHLERIR EKLAENIHEL WALTRIEQGW TYGPVRDDN KRLHPCLVNF HSLPEPERNY NLQMSGETLK TLLALGCHVG MADEKAEDNL KKTKLPKTYM MSNGYKPAPL D LSHVRLTP AQTTLVDRLA ENGHNVWARD RVAQGWSYSA VQDIPARRNP RLVPYRLLDE ATKRSNRDSL CQAVRTLLGY GY NIEPPDQ EPSQVENQSR WDRVRIFRAE KSYTVQSGRW YFEFEAVTTG EMRVGWARPE LRPDVELGAD ELAYVFNGHR GQR WHLGSE PFGRPWQSGD VVGCMIDLTE NTIIFTLNGE VLMSDSGSET AFREIEIGDG FLPVCSLGPG QVGHLNLGQD VSSL RFFAI CGLQEGFEPF AINMQRPVTT WFSKSLPQFE PVPPEHPHYE VARMDGTVDT PPCLRLAHR(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)MPLS AAMFLSERKN PAPQCPPRLE VQMLMP VSW SRMPNHFLQV ETRRAGERLG WAVQCQDPLT MMALHIPEEN RCMDILELSE RLDLQRFHSH TLRLYRAVCA LGNNRVA HA LCSHVDQAQL LHALEDAHLP GPLRAGYYDL LISIHLESAC RSRRSMLSEY IVPLTPETRA ITLFPPGRKG GNARRHGL P GVGVTTSLRP PHHFSPPCFV AALPAAGVAE APARLSPAIP LEALRDKALR MLGEAVRDGG QHARDPVGGS VEFQFVPVL KLVSTLLVMG IFGDEDVKQI LKMIEPEVFT EEEEEEEEEE EEEEEEEEDE EEKEEDEEEE EKEDAEKEEE EAPEGEKEDL EEGLLQMKL PESVKLQMCN LLEYFCDQEL QHRVESLAAF AERYVDKLQA NQRSRYALLM RAFTMSAAET ARRTREFRSP P QEQINMLL HFKDEADEED CPLPEDIRQD LQDFHQDLLA HCGIQLEGEE EEPEEETSLS SRLRSLLETV RLVKKKEEKP EE ELPAEEK KPQSLQELVS HMVVRWAQED YVQSPELVRA MFSLLHRQYD GLGELLRALP RAYTISPSSV EDTMSLLECL GQI RSLLIV QMGPQEENLM IQSIGNIMNN KVFYQHPNLM RALGMHETVM EVMVNVLGGG ETKEIRFPKM VTSCCRFLCY FCRI SRQNQ RSMFDHLSYL LENSGIGLGM QGSTPLDVAA ASVIDNNELA LALQEQDLEK VVSYLAGCGL QSCPMLLAKG YPDIG WNPC GGERYLDFLR FAVFVNGESV EENANVVVRL LIRKPECFGP ALRGEGGSGL LAAIEEAIRI SEDPARDGPG VRRDRR REH FGEEPPEENR VHLGHAIMSF YAALIDLLGR CAPEMHLIQA GKGEALRIRA ILRSLVPLDD LVGIISLPLQ IPTL (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)NFDPR PVETLNVIIP EKLDSFINKF AEYTHEKWAF DKIQNNWSY GENVDEELKT HPMLRPYKTF SEKDKEIYRW PIKESLKAMI AWEWTIEKAR EGEEERTEKK KTRKISQTAQ T YDPREGYN PQPPDLSGVT LSRELQAMAE QLAENYHNTW GRKKKQELEA KGGGTHPLLV PYDTLTAKEK ARDREKAQEL LK FLQMNGY AVTR(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK) (UNK)TPLYNLP THRACNMFLE SYKAAWILTE DHSFEDRMID DLSKAGEQEE EEEEVEEKKP DPLHQLVLHF SRTALTEK S KLDEDYLYMA YADIMAKSCH LEEGGENGEA EEEEVEVSFE EKEMEKQRLL YQQSRLHTRG AAEMVLQMIS ACKGETGAM VSSTLKLGIS ILNGGNAEVQ QKMLDYLKDK KEVGFFQSIQ ALMQTCSVLD LNAFERQNKA EGLGMVNEDG TVINRQNGEK VMADDEFTQ DLFRFLQLLC EGHNNDFQNY LRTQTGNTTT INIIICTVDY LLRLQESISD FYWYYSGKDV IEEQGKRNFS K AMSVAKQV FNSLTEYIQG PCTGNQQSLA HSRLWDAVVG FLHVFAHMMM KLAQDSSQIE LLKELLDLQK DMVVMLLSLL EG NVVNGMI ARQMVDMLVE SSSNVEMILK FFDMFLKLKD IVGSEAFQDY VTDPRGLISK KDFQKAMDSQ KQFTGPEIQF LLS CSEADE NEMINFEEFA NRFQEPARDI GFNVAVLLTN LSEHVPHDPR LRNFLELAES ILEYFRPYLG RIEIMGASRR IERI YFEIS ETNRAQWEMP QVKESKRQFI FDVVNEGGEA EKMELFVSFC EDTIFEMQIA AQISE(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)FWGELEV QRVKFLNYLS RNFYTLRFLA LFLAFAINFI LLFYKVSDSP PGE DDMEGS AAGDLAGAGS GGGSGWGSGA GEEAEGDEDE NMVYYFLEES TGYMEPALWC LSLLHTLVAF LCIIGYNCLK VPLV IFKRE KELARKLEFD GLYITEQPGD DDVKGQWDRL VLNTPSFPSN YWDKFVKRKV LDKHGDIFGR ERIAELLGMD LASLE ITAH NERKPDPPPG LLTWLMSIDV KYQIWKFGVI FTDNSFLYLG WYMVMSLLGH YNNFFFAAHL LDIAMGVKTL RTILSS VTH NGKQLVMTVG LLAVVVYLYT VVAFNFFRKF YNKSEDEDEP DMKCDDMMTC YLFHMYVGVR AGGGIGDEIE DPAGDEY EL YRVVFDITFF FFVIVILLAI IQGLIIDAFG ELRDQQEQVK EDMETKCFIC GIGSDYFDTT PHGFETHTLE EHNLANYM F FLMYLINKDE TEHTGQESYV WKMYQERCWD FFPAGDCFRK QYEDQLS UniProtKB: Ryanodine receptor 1, Ryanodine receptor 1, Ryanodine receptor 1, Ryanodine receptor 1, Ryanodine receptor 1 |
-分子 #3: ADENOSINE-5'-TRIPHOSPHATE
| 分子 | 名称: ADENOSINE-5'-TRIPHOSPHATE / タイプ: ligand / ID: 3 / コピー数: 4 / 式: ATP |
|---|---|
| 分子量 | 理論値: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-分子 #4: CAFFEINE
| 分子 | 名称: CAFFEINE / タイプ: ligand / ID: 4 / コピー数: 4 / 式: CFF |
|---|---|
| 分子量 | 理論値: 194.191 Da |
| Chemical component information |  ChemComp-CFF: |
-分子 #5: ZINC ION
| 分子 | 名称: ZINC ION / タイプ: ligand / ID: 5 / コピー数: 4 / 式: ZN |
|---|---|
| 分子量 | 理論値: 65.409 Da |
-分子 #6: CALCIUM ION
| 分子 | 名称: CALCIUM ION / タイプ: ligand / ID: 6 / コピー数: 4 / 式: CA |
|---|---|
| 分子量 | 理論値: 40.078 Da |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 6.0 mg/mL |
|---|---|
| 緩衝液 | pH: 7.4 |
| グリッド | モデル: Quantifoil / 材質: GOLD / メッシュ: 400 |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 277 K / 装置: FEI VITROBOT MARK IV 詳細: Blotted for 3-4 seconds on both sides with Whatman ashless filter paper, blot force 3, wait time 30 seconds. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI POLARA 300 |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 検出モード: COUNTING / 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD |
| 実験機器 |  モデル: Tecnai Polara / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)