[English] 日本語
 Yorodumi
Yorodumi- EMDB-8302: HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8302 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 | |||||||||
 Map data Map data | HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
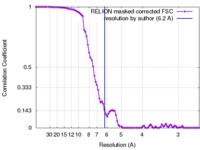
| Method | single particle reconstruction / cryo EM / Resolution: 6.2 Å | |||||||||
 Authors Authors | Pallesen J / de Val N / Ozorowski G / Cottrell CA / Ward AB | |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2016 Journal: Nat Microbiol / Year: 2016Title: An HIV-1 antibody from an elite neutralizer implicates the fusion peptide as a site of vulnerability. Authors: Marit J van Gils / Tom L G M van den Kerkhof / Gabriel Ozorowski / Christopher A Cottrell / Devin Sok / Matthias Pauthner / Jesper Pallesen / Natalia de Val / Anila Yasmeen / Steven W de ...Authors: Marit J van Gils / Tom L G M van den Kerkhof / Gabriel Ozorowski / Christopher A Cottrell / Devin Sok / Matthias Pauthner / Jesper Pallesen / Natalia de Val / Anila Yasmeen / Steven W de Taeye / Anna Schorcht / Stephanie Gumbs / Inez Johanna / Karen Saye-Francisco / Chi-Hui Liang / Elise Landais / Xiaoyan Nie / Laura K Pritchard / Max Crispin / Garnett Kelsoe / Ian A Wilson / Hanneke Schuitemaker / Per Johan Klasse / John P Moore / Dennis R Burton / Andrew B Ward / Rogier W Sanders /    Abstract: The induction by vaccination of broadly neutralizing antibodies (bNAbs) capable of neutralizing various HIV-1 viral strains is challenging, but understanding how a subset of HIV-infected individuals ...The induction by vaccination of broadly neutralizing antibodies (bNAbs) capable of neutralizing various HIV-1 viral strains is challenging, but understanding how a subset of HIV-infected individuals develops bNAbs may guide immunization strategies. Here, we describe the isolation and characterization of the bNAb ACS202 from an elite neutralizer that recognizes a new, trimer-specific and cleavage-dependent epitope at the gp120-gp41 interface of the envelope glycoprotein (Env), involving the glycan N88 and the gp41 fusion peptide. In addition, an Env trimer, AMC011 SOSIP.v4.2, based on early virus isolates from the same elite neutralizer, was constructed, and its structure by cryo-electron microscopy at 6.2 Å resolution reveals a closed, pre-fusion conformation similar to that of the BG505 SOSIP.664 trimer. The availability of a native-like Env trimer and a bNAb from the same elite neutralizer provides the opportunity to design vaccination strategies aimed at generating similar bNAbs against a key functional site on HIV-1. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8302.map.gz emd_8302.map.gz | 59.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8302-v30.xml emd-8302-v30.xml emd-8302.xml emd-8302.xml | 23.4 KB 23.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8302_fsc.xml emd_8302_fsc.xml | 8.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_8302.png emd_8302.png | 172.8 KB | ||
| Others |  emd_8302_additional.map.gz emd_8302_additional.map.gz emd_8302_half_map_1.map.gz emd_8302_half_map_1.map.gz emd_8302_half_map_2.map.gz emd_8302_half_map_2.map.gz | 59.7 MB 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8302 http://ftp.pdbj.org/pub/emdb/structures/EMD-8302 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8302 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8302 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8302.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8302.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Additional map: HIV Clade B Env SOSIP ectodomain...
| File | emd_8302_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Additional map: HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map #1: HIV Clade B Env SOSIP...
| File | emd_8302_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map #1: HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map #2: HIV Clade B Env SOSIP...
| File | emd_8302_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map #2: HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort...
| Entire | Name: HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 |
|---|---|
| Components |
|
-Supramolecule #1: HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort...
| Supramolecule | Name: HIV Clade B Env SOSIP ectodomain trimer from the Amsterdam Cohort Study patient AMC011 in complex with Fab from IgG PGV04 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 600 KDa |
-Macromolecule #1: PGV04 Fab HC
| Macromolecule | Name: PGV04 Fab HC / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLVQSGSG VKKPGASVRV SCWTSEDIFE RTELIHWVRQ APGQGLEWIG WVKTVTGAVN FGSPDFRQRV SLTRDRDLFT AHMDIRGLTQ GDTATYFCAR QKFYTGGQGW YFDLWGRGTL IVVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE PVTVSWNSGA ...String: QVQLVQSGSG VKKPGASVRV SCWTSEDIFE RTELIHWVRQ APGQGLEWIG WVKTVTGAVN FGSPDFRQRV SLTRDRDLFT AHMDIRGLTQ GDTATYFCAR QKFYTGGQGW YFDLWGRGTL IVVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSC |
-Macromolecule #2: PGV04 Fab LC
| Macromolecule | Name: PGV04 Fab LC / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EIVLTQSPGT LSLSPGETAS LSCTAASYGH MTWYQKKPGQ PPKLLIFATS KRASGIPDRF SGSQFGKQYT LTITRMEPED FARYYCQQLE FFGQGTRLEI RRTVAAPSVF IFPPSDEQLK SGTASVVCLL NNFYPREAKV QWKVDNALQS GNSQESVTEQ DSKDSTYSLS ...String: EIVLTQSPGT LSLSPGETAS LSCTAASYGH MTWYQKKPGQ PPKLLIFATS KRASGIPDRF SGSQFGKQYT LTITRMEPED FARYYCQQLE FFGQGTRLEI RRTVAAPSVF IFPPSDEQLK SGTASVVCLL NNFYPREAKV QWKVDNALQS GNSQESVTEQ DSKDSTYSLS STLTLSKADY EKHKVYACEV THQGLSSPVT KSFNRGEC |
-Macromolecule #3: AMC011v.4.2_SOSIP
| Macromolecule | Name: AMC011v.4.2_SOSIP / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARAEQLW VTVYYGVPVW KEATTTLFCA SDARAYDTEV RNVWATHACV PTDPNPQEVV LENVTENFNM WKNNMVEQMH EDIISLWDQS LKPCVKLTPL CVTLNCTDLR NATNTNATNT TSSSRGTMEG GEIKNCSFNI ...String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARAEQLW VTVYYGVPVW KEATTTLFCA SDARAYDTEV RNVWATHACV PTDPNPQEVV LENVTENFNM WKNNMVEQMH EDIISLWDQS LKPCVKLTPL CVTLNCTDLR NATNTNATNT TSSSRGTMEG GEIKNCSFNI TTSMRDKVQK EYALFYKLDV VPIKNDNTSY RLISCNTSVI TQACPKVSFE PIPIHYCAPA GFAILKCNDK KFNGTGPCTN VSTVQCTHGI RPVVSTQLLL NGSLAEEEVV IRSANFTDNA KIIIVQLNKS VEINCTRPNN NTRKSIHIGP GRWFYTTGEI IGDIRQAHCN ISGTKWNDTL KQIVVKLKEQ FGNKTIVFNH SSGGDPEIVM HSFNCGGEFF YCNSTQLFNS TWNDGSNYTG TIVLPCRIKQ IVNMWQEVGK AMYAPPIKGQ IRCSSNITGL ILIRDGGKNR SENTEIFRPG GGDMRDNWRS ELYKYKVVKI EPLGIAPTKC KRRVVQRRRR RRAVGIGAVF LGFLGAAGST MGAASMTLTV QARQLLSGIV QQQNNLLRAP EAQQHLLKLT VWGIKQLQAR VLAVERYLKD QQLLGIWGCS GKLICCTAVP WNTSWSNKSY NQIWNNMTWM EWEREIDNYT SLIYTLIEDS QNQQEKNEQE LLELD |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.71 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Component - Formula: C4H11NO3 / Component - Name: Tris Details: DDM was added to a concentration of 0.3 mM immediately before freezing. |
| Grid | Model: Electron Microscopy Sciences, C-flat / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Instrument: HOMEMADE PLUNGER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 77.0 K / Max: 77.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Sampling interval: 5.0 µm / Digitization - Frames/image: 1-35 / Number grids imaged: 1 / Number real images: 3480 / Average exposure time: 7.0 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: MolProbity/RMSD |
|---|
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)