[English] 日本語
 Yorodumi
Yorodumi- EMDB-20735: Cryo-EM structure of CH235UCA bound to Man5-enriched CH505.N279K.... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20735 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of CH235UCA bound to Man5-enriched CH505.N279K.G458Y.SOSIP.664 | |||||||||
 Map data Map data | Refined and sharpened map from Cryosparc | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | CH235 lineage / CH505 virus / vaccine design / cryo-EM / VIRAL PROTEIN / VIRAL PROTEIN-Immune System complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell ...symbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Acharya P | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2019 Journal: PLoS Pathog / Year: 2019Title: Neutralization-guided design of HIV-1 envelope trimers with high affinity for the unmutated common ancestor of CH235 lineage CD4bs broadly neutralizing antibodies. Authors: Celia C LaBranche / Rory Henderson / Allen Hsu / Shay Behrens / Xuejun Chen / Tongqing Zhou / Kevin Wiehe / Kevin O Saunders / S Munir Alam / Mattia Bonsignori / Mario J Borgnia / Quentin J ...Authors: Celia C LaBranche / Rory Henderson / Allen Hsu / Shay Behrens / Xuejun Chen / Tongqing Zhou / Kevin Wiehe / Kevin O Saunders / S Munir Alam / Mattia Bonsignori / Mario J Borgnia / Quentin J Sattentau / Amanda Eaton / Kelli Greene / Hongmei Gao / Hua-Xin Liao / Wilton B Williams / James Peacock / Haili Tang / Lautaro G Perez / Robert J Edwards / Thomas B Kepler / Bette T Korber / Peter D Kwong / John R Mascola / Priyamvada Acharya / Barton F Haynes / David C Montefiori /   Abstract: The CD4 binding site (CD4bs) of the HIV-1 envelope glycoprotein is susceptible to multiple lineages of broadly neutralizing antibodies (bnAbs) that are attractive to elicit with vaccines. The CH235 ...The CD4 binding site (CD4bs) of the HIV-1 envelope glycoprotein is susceptible to multiple lineages of broadly neutralizing antibodies (bnAbs) that are attractive to elicit with vaccines. The CH235 lineage (VH1-46) of CD4bs bnAbs is particularly attractive because the most mature members neutralize 90% of circulating strains, do not possess long HCDR3 regions, and do not contain insertions and deletions that may be difficult to induce. We used virus neutralization to measure the interaction of CH235 unmutated common ancestor (CH235 UCA) with functional Env trimers on infectious virions to guide immunogen design for this bnAb lineage. Two Env mutations were identified, one in loop D (N279K) and another in V5 (G458Y), that acted synergistically to render autologous CH505 transmitted/founder virus susceptible to neutralization by CH235 UCA. Man5-enriched N-glycans provided additional synergy for neutralization. CH235 UCA bound with nanomolar affinity to corresponding soluble native-like Env trimers as candidate immunogens. A cryo-EM structure of CH235 UCA bound to Man5-enriched CH505.N279K.G458Y.SOSIP.664 revealed interactions of the antibody light chain complementarity determining region 3 (CDR L3) with the engineered Env loops D and V5. These results demonstrate that virus neutralization can directly inform vaccine design and suggest a germline targeting and reverse engineering strategy to initiate and mature the CH235 bnAb lineage. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20735.map.gz emd_20735.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20735-v30.xml emd-20735-v30.xml emd-20735.xml emd-20735.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20735.png emd_20735.png | 111.6 KB | ||
| Filedesc metadata |  emd-20735.cif.gz emd-20735.cif.gz | 6.9 KB | ||
| Others |  emd_20735_additional.map.gz emd_20735_additional.map.gz | 112.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20735 http://ftp.pdbj.org/pub/emdb/structures/EMD-20735 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20735 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20735 | HTTPS FTP |
-Related structure data
| Related structure data |  6udaMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20735.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20735.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined and sharpened map from Cryosparc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
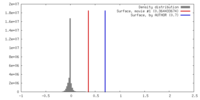
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Refined map from cryosparc sharpened in Phenix
| File | emd_20735_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined map from cryosparc sharpened in Phenix | ||||||||||||
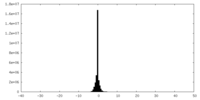
| Projections & Slices |
| ||||||||||||
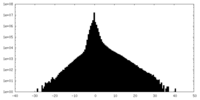
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of the CH235 unmutated common ancestor (UCA) bound to Man...
| Entire | Name: Complex of the CH235 unmutated common ancestor (UCA) bound to Man5-enriched CH505.N279K.G458Y.SOSIP.664 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of the CH235 unmutated common ancestor (UCA) bound to Man...
| Supramolecule | Name: Complex of the CH235 unmutated common ancestor (UCA) bound to Man5-enriched CH505.N279K.G458Y.SOSIP.664 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #2-#4 |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 360 KDa |
-Macromolecule #1: CH505.N279K.G458Y.SOSIP.664 gp120
| Macromolecule | Name: CH505.N279K.G458Y.SOSIP.664 gp120 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 54.546402 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MPMGSLQPLA TLYLLGMLVA SVLAAENLWV TVYYGVPVWK EAKTTLFCAS DAKAYEKEVH NVWATHACVP TDPNPQEMVL KNVTENFNM WKNDMVDQMH EDVISLWDQS LKPCVKLTPL CVTLNCTNAT ASNSSIIEGM KNCSFNITTE LRDKREKKNA L FYKLDIVQ ...String: MPMGSLQPLA TLYLLGMLVA SVLAAENLWV TVYYGVPVWK EAKTTLFCAS DAKAYEKEVH NVWATHACVP TDPNPQEMVL KNVTENFNM WKNDMVDQMH EDVISLWDQS LKPCVKLTPL CVTLNCTNAT ASNSSIIEGM KNCSFNITTE LRDKREKKNA L FYKLDIVQ LDGNSSQYRL INCNTSVITQ ACPKVSFDPI PIHYCAPAGY AILKCNNKTF TGTGPCNNVS TVQCTHGIKP VV STQLLLN GSLAEGEIII RSENITKNVK TIIVHLNESV KIECTRPNNK TRTSIRIGPG QAFYATGQVI GDIREAYCNI NES KWNETL QRVSKKLKEY FPHKNITFQP SSGGDLEITT HSFNCGGEFF YCNTSSLFNR TYMANSTDMA NSTETNSTRT ITIH CRIKQ IINMWQEVGR AMYAPPIAGN ITCISNITGL LLTRDYGKNN TETFRPGGGN MKDNWRSELY KYKVVKIEPL GVAPT RCKR RVV UniProtKB: Envelope glycoprotein gp160 |
-Macromolecule #2: CH235 UCA heavy chain Fab
| Macromolecule | Name: CH235 UCA heavy chain Fab / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.635938 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: METDTLLLWV LLLWVPGSTG DQVQLVQSGA EVKKPGASVK VSCKASGYTF TSYYMHWVRQ APGQGLEWMG IINPSGGSTS YAQKFQGRV TMTRDTSTST VYMELSSLRS EDTAVYYCAR DVGTEGSLLH FDYWGQGTLV TVSSASTKGP SVFPLAPSSK S TSGGTAAL ...String: METDTLLLWV LLLWVPGSTG DQVQLVQSGA EVKKPGASVK VSCKASGYTF TSYYMHWVRQ APGQGLEWMG IINPSGGSTS YAQKFQGRV TMTRDTSTST VYMELSSLRS EDTAVYYCAR DVGTEGSLLH FDYWGQGTLV TVSSASTKGP SVFPLAPSSK S TSGGTAAL GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KR VEPKSCD KT |
-Macromolecule #3: CH505.N279K.G458Y.SOSIP.664 gp41
| Macromolecule | Name: CH505.N279K.G458Y.SOSIP.664 gp41 / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 18.146699 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GRRRRRRAVG IGAVFLGFLG AAGSTMGAAS MTLTVQARNL LSGIVQQQSN LLRAPEAQQH LLKLTVWGIK QLQARVLAVE RYLRDQQLL GIWGCSGKLI CCTNVPWNSS WSNRNLSEIW DNMTWLQWDK EISNYTQIIY GLLEESQNQQ EKNEQDLLAL D UniProtKB: Envelope glycoprotein gp160 |
-Macromolecule #4: CH235 UCA light chain Fab
| Macromolecule | Name: CH235 UCA light chain Fab / type: protein_or_peptide / ID: 4 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.65957 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: METDTLLLWV LLLWVPGSTG DEIVMTQSPA TLSVSPGERA TLSCRASQSV SSNLAWYQQK PGQAPRLLIY GASTRATGIP ARFSGSGSG TEFTLTISSL QSEDFAVYYC QQYNNWWTFG QGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF Y PREAKVQW ...String: METDTLLLWV LLLWVPGSTG DEIVMTQSPA TLSVSPGERA TLSCRASQSV SSNLAWYQQK PGQAPRLLIY GASTRATGIP ARFSGSGSG TEFTLTISSL QSEDFAVYYC QQYNNWWTFG QGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF Y PREAKVQW KVDNALQSGN SQESVTEQDS KDSTYSLSST LTLSKADYEK HKVYACEVTH QGLSSPVTKS FNRGEC |
-Macromolecule #6: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 6 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL |
|---|---|
| Buffer | pH: 7.2 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 42.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Number classes used: 1 / Applied symmetry - Point group: C3 (3 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 4.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 22093 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)