+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8216 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | MicroED structure of tau VQIVYK peptide at 1.1 A resolution | |||||||||
 Map data Map data | tau VQIVYK peptide | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Amyloid / PROTEIN FIBRIL | |||||||||
| Function / homology |  Function and homology information Function and homology informationplus-end-directed organelle transport along microtubule / histone-dependent DNA binding / negative regulation of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / phosphatidylinositol bisphosphate binding / generation of neurons ...plus-end-directed organelle transport along microtubule / histone-dependent DNA binding / negative regulation of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / phosphatidylinositol bisphosphate binding / generation of neurons / rRNA metabolic process / axonal transport of mitochondrion / regulation of mitochondrial fission / axon development / regulation of microtubule-based movement / regulation of chromosome organization / central nervous system neuron development / intracellular distribution of mitochondria / minor groove of adenine-thymine-rich DNA binding / lipoprotein particle binding / microtubule polymerization / negative regulation of mitochondrial membrane potential / regulation of microtubule polymerization / dynactin binding / apolipoprotein binding / main axon / protein polymerization / axolemma / Caspase-mediated cleavage of cytoskeletal proteins / regulation of microtubule polymerization or depolymerization / negative regulation of mitochondrial fission / glial cell projection / neurofibrillary tangle assembly / positive regulation of axon extension / regulation of cellular response to heat / Activation of AMPK downstream of NMDARs / positive regulation of superoxide anion generation / positive regulation of protein localization / regulation of long-term synaptic depression / cellular response to brain-derived neurotrophic factor stimulus / positive regulation of microtubule polymerization / supramolecular fiber organization / synapse assembly / cytoplasmic microtubule organization / regulation of calcium-mediated signaling / somatodendritic compartment / axon cytoplasm / phosphatidylinositol binding / astrocyte activation / enzyme inhibitor activity / nuclear periphery / stress granule assembly / protein phosphatase 2A binding / regulation of microtubule cytoskeleton organization / cellular response to reactive oxygen species / Hsp90 protein binding / microglial cell activation / cellular response to nerve growth factor stimulus / synapse organization / PKR-mediated signaling / regulation of synaptic plasticity / protein homooligomerization / regulation of autophagy / response to lead ion / SH3 domain binding / microtubule cytoskeleton organization / memory / cytoplasmic ribonucleoprotein granule / neuron projection development / cell-cell signaling / single-stranded DNA binding / protein-folding chaperone binding / cellular response to heat / microtubule cytoskeleton / actin binding / growth cone / cell body / double-stranded DNA binding / protein-macromolecule adaptor activity / microtubule binding / sequence-specific DNA binding / dendritic spine / amyloid fibril formation / microtubule / learning or memory / neuron projection / membrane raft / negative regulation of gene expression / axon / neuronal cell body / DNA damage response / dendrite / protein kinase binding / enzyme binding / mitochondrion / DNA binding / RNA binding / extracellular region / identical protein binding / nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | electron crystallography / cryo EM / Resolution: 1.1 Å | |||||||||
 Authors Authors | de la Cruz MJ / Hattne J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Methods / Year: 2017 Journal: Nat Methods / Year: 2017Title: Atomic-resolution structures from fragmented protein crystals with the cryoEM method MicroED. Authors: M Jason de la Cruz / Johan Hattne / Dan Shi / Paul Seidler / Jose Rodriguez / Francis E Reyes / Michael R Sawaya / Duilio Cascio / Simon C Weiss / Sun Kyung Kim / Cynthia S Hinck / Andrew P ...Authors: M Jason de la Cruz / Johan Hattne / Dan Shi / Paul Seidler / Jose Rodriguez / Francis E Reyes / Michael R Sawaya / Duilio Cascio / Simon C Weiss / Sun Kyung Kim / Cynthia S Hinck / Andrew P Hinck / Guillermo Calero / David Eisenberg / Tamir Gonen /  Abstract: Traditionally, crystallographic analysis of macromolecules has depended on large, well-ordered crystals, which often require significant effort to obtain. Even sizable crystals sometimes suffer from ...Traditionally, crystallographic analysis of macromolecules has depended on large, well-ordered crystals, which often require significant effort to obtain. Even sizable crystals sometimes suffer from pathologies that render them inappropriate for high-resolution structure determination. Here we show that fragmentation of large, imperfect crystals into microcrystals or nanocrystals can provide a simple path for high-resolution structure determination by the cryoEM method MicroED and potentially by serial femtosecond crystallography. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8216.map.gz emd_8216.map.gz | 1.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8216-v30.xml emd-8216-v30.xml emd-8216.xml emd-8216.xml | 13 KB 13 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8216.png emd_8216.png | 156.4 KB | ||
| Filedesc metadata |  emd-8216.cif.gz emd-8216.cif.gz | 4.7 KB | ||
| Filedesc structureFactors |  emd_8216_sf.cif.gz emd_8216_sf.cif.gz | 45.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8216 http://ftp.pdbj.org/pub/emdb/structures/EMD-8216 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8216 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8216 | HTTPS FTP |
-Related structure data
| Related structure data |  5k7nMC  8217C  8218C  8219C  8220C  8221C  8222C  8472C  5k7oC  5k7pC  5k7qC  5k7rC  5k7sC  5k7tC 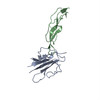 5ty4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8216.map.gz / Format: CCP4 / Size: 1.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8216.map.gz / Format: CCP4 / Size: 1.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | tau VQIVYK peptide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
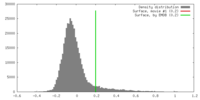
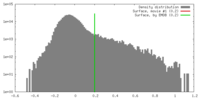
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X: 0.36775 Å / Y: 0.31188 Å / Z: 0.3574 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : VQIVYK
| Entire | Name: VQIVYK |
|---|---|
| Components |
|
-Supramolecule #1: VQIVYK
| Supramolecule | Name: VQIVYK / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 747 Da |
-Macromolecule #1: VQIVYK
| Macromolecule | Name: VQIVYK / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 749.917 Da |
| Sequence | String: VQIVYK |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 2 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron crystallography |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Buffer | pH: 8.5 / Component:
| ||||||
|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Image recording | Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Digitization - Dimensions - Width: 2048 pixel / Digitization - Dimensions - Height: 2048 pixel / Number grids imaged: 1 / Number real images: 299 / Number diffraction images: 299 / Average exposure time: 2.1 sec. / Average electron dose: 0.002 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DIFFRACTION / Camera length: 730 mm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 1.1 Å / Resolution method: DIFFRACTION PATTERN/LAYERLINES |
|---|---|
| Merging software list | Software - Name: XDS (ver. May 1, 2016) |
| Crystallography statistics | Number intensities measured: 6185 / Number structure factors: 3319 / Fourier space coverage: 83 / R sym: 12.9 / R merge: 12.9 / Overall phase error: 0 / Overall phase residual: 39.4 / Phase error rejection criteria: 0 / High resolution: 1.1 Å / Shell - Shell ID: 1 / Shell - High resolution: 1.1 Å / Shell - Low resolution: 1.23 Å / Shell - Number structure factors: 255 / Shell - Phase residual: 47.6 / Shell - Fourier space coverage: 79.4 / Shell - Multiplicity: 1.8 |
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-5k7n: |
 Movie
Movie Controller
Controller









 Y (Sec.)
Y (Sec.) X (Row.)
X (Row.) Z (Col.)
Z (Col.)