[English] 日本語
 Yorodumi
Yorodumi- EMDB-7462: Insulin Receptor ectodomain in complex with two insulin molecules -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7462 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Insulin Receptor ectodomain in complex with two insulin molecules | ||||||||||||
 Map data Map data | Insulin Receptor ectodomain in complex with two insulin molecules | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | signaling / SIGNALING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to palmitoleic acid / response to L-arginine / regulation of female gonad development / positive regulation of meiotic cell cycle / insulin-like growth factor II binding / positive regulation of developmental growth / male sex determination / insulin receptor complex / positive regulation of protein-containing complex disassembly / insulin-like growth factor I binding ...cellular response to palmitoleic acid / response to L-arginine / regulation of female gonad development / positive regulation of meiotic cell cycle / insulin-like growth factor II binding / positive regulation of developmental growth / male sex determination / insulin receptor complex / positive regulation of protein-containing complex disassembly / insulin-like growth factor I binding / insulin receptor activity / exocrine pancreas development / dendritic spine maintenance / cargo receptor activity / insulin binding / adrenal gland development / negative regulation of glycogen catabolic process / PTB domain binding / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / neuronal cell body membrane / negative regulation of feeding behavior / Signaling by Insulin receptor / IRS activation / regulation of protein secretion / Insulin processing / positive regulation of peptide hormone secretion / positive regulation of respiratory burst / negative regulation of acute inflammatory response / Regulation of gene expression in beta cells / amyloid-beta clearance / alpha-beta T cell activation / positive regulation of receptor internalization / insulin receptor substrate binding / regulation of embryonic development / positive regulation of dendritic spine maintenance / Synthesis, secretion, and deacylation of Ghrelin / epidermis development / negative regulation of protein secretion / negative regulation of gluconeogenesis / positive regulation of glycogen biosynthetic process / fatty acid homeostasis / Signal attenuation / protein kinase activator activity / positive regulation of insulin receptor signaling pathway / negative regulation of respiratory burst involved in inflammatory response / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / negative regulation of lipid catabolic process / positive regulation of lipid biosynthetic process / heart morphogenesis / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / regulation of protein localization to plasma membrane / transport across blood-brain barrier / phosphatidylinositol 3-kinase binding / nitric oxide-cGMP-mediated signaling / transport vesicle / COPI-mediated anterograde transport / positive regulation of nitric-oxide synthase activity / Insulin receptor recycling / negative regulation of reactive oxygen species biosynthetic process / positive regulation of brown fat cell differentiation / insulin-like growth factor receptor binding / NPAS4 regulates expression of target genes / dendrite membrane / neuron projection maintenance / endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of mitotic nuclear division / receptor-mediated endocytosis / Insulin receptor signalling cascade / positive regulation of glycolytic process / positive regulation of cytokine production / endosome lumen / positive regulation of long-term synaptic potentiation / acute-phase response / learning / positive regulation of D-glucose import across plasma membrane / positive regulation of protein secretion / insulin receptor binding / positive regulation of cell differentiation / Regulation of insulin secretion / wound healing / receptor protein-tyrosine kinase / positive regulation of neuron projection development / hormone activity / regulation of synaptic plasticity / negative regulation of protein catabolic process / caveola / receptor internalization / positive regulation of protein localization to nucleus / Golgi lumen / cellular response to growth factor stimulus / vasodilation / male gonad development / cognition / memory / cellular response to insulin stimulus / glucose metabolic process / positive regulation of nitric oxide biosynthetic process / insulin receptor signaling pathway / late endosome Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||
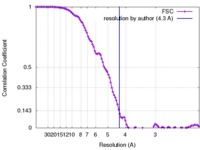
| Method | single particle reconstruction / cryo EM / Resolution: 4.3 Å | ||||||||||||
 Authors Authors | Scapin G / Dandey VP | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Structure of the insulin receptor-insulin complex by single-particle cryo-EM analysis. Authors: Giovanna Scapin / Venkata P Dandey / Zhening Zhang / Winifred Prosise / Alan Hruza / Theresa Kelly / Todd Mayhood / Corey Strickland / Clinton S Potter / Bridget Carragher /  Abstract: The insulin receptor is a dimeric protein that has a crucial role in controlling glucose homeostasis, regulating lipid, protein and carbohydrate metabolism, and modulating brain neurotransmitter ...The insulin receptor is a dimeric protein that has a crucial role in controlling glucose homeostasis, regulating lipid, protein and carbohydrate metabolism, and modulating brain neurotransmitter levels. Insulin receptor dysfunction has been associated with many diseases, including diabetes, cancer and Alzheimer's disease. The primary sequence of the receptor has been known since the 1980s, and is composed of an extracellular portion (the ectodomain, ECD), a single transmembrane helix and an intracellular tyrosine kinase domain. Binding of insulin to the dimeric ECD triggers auto-phosphorylation of the tyrosine kinase domain and subsequent activation of downstream signalling molecules. Biochemical and mutagenesis data have identified two putative insulin-binding sites, S1 and S2. The structures of insulin bound to an ECD fragment containing S1 and of the apo ectodomain have previously been reported, but details of insulin binding to the full receptor and the signal propagation mechanism are still not understood. Here we report single-particle cryo-electron microscopy reconstructions of the 1:2 (4.3 Å) and 1:1 (7.4 Å) complexes of the insulin receptor ECD dimer with insulin. The symmetrical 4.3 Å structure shows two insulin molecules per dimer, each bound between the leucine-rich subdomain L1 of one monomer and the first fibronectin-like domain (FnIII-1) of the other monomer, and making extensive interactions with the α-subunit C-terminal helix (α-CT helix). The 7.4 Å structure has only one similarly bound insulin per receptor dimer. The structures confirm the binding interactions at S1 and define the full S2 binding site. These insulin receptor states suggest that recruitment of the α-CT helix upon binding of the first insulin changes the relative subdomain orientations and triggers downstream signal propagation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7462.map.gz emd_7462.map.gz | 85.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7462-v30.xml emd-7462-v30.xml emd-7462.xml emd-7462.xml | 17.3 KB 17.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_7462_fsc.xml emd_7462_fsc.xml | 12 KB | Display |  FSC data file FSC data file |
| Images |  emd_7462.png emd_7462.png | 98.6 KB | ||
| Filedesc metadata |  emd-7462.cif.gz emd-7462.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7462 http://ftp.pdbj.org/pub/emdb/structures/EMD-7462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7462 | HTTPS FTP |
-Related structure data
| Related structure data |  6ce9MC  7461C  7463C  6ce7C  6cebC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7462.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7462.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Insulin Receptor ectodomain in complex with two insulin molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Insulin Receptor Ectodomain in complex with two insulin molecules
+Supramolecule #1: Insulin Receptor Ectodomain in complex with two insulin molecules
+Supramolecule #2: Insulin receptor
+Supramolecule #3: Insulin receptor subunit alpha
+Supramolecule #4: Insulin A chain
+Supramolecule #5: Insulin B chain
+Macromolecule #1: Insulin receptor
+Macromolecule #2: Insulin receptor
+Macromolecule #3: Insulin A chain
+Macromolecule #4: Insulin B chain
+Macromolecule #9: 2-acetamido-2-deoxy-beta-D-glucopyranose
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: Hepes Saline (HBS) |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 293 K / Instrument: HOMEMADE PLUNGER / Details: Grids made with SpotItOn. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average electron dose: 45.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-6ce9: |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)