[English] 日本語
 Yorodumi
Yorodumi- EMDB-7437: Single-Particle reconstruction of DARP14 - A designed protein sca... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7437 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Single-Particle reconstruction of DARP14 - A designed protein scaffold displaying ~17kDa DARPin proteins | |||||||||
 Map data Map data | Single-Particle reconstruction of DARP14 - A designed protein scaffold displaying ~17kDa DARPin proteins | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Designed Complex / DARPin / Scaffold / Single-Particle Cryo-EM / DE NOVO PROTEIN | |||||||||
| Function / homology | 5-carboxymethyl-2-hydroxymuconate isomerase / 5-carboxymethyl-2-hydroxymuconate isomerase / 5-carboxymethyl-2-hydroxymuconate delta-isomerase activity / Tautomerase/MIF superfamily / 5-carboxymethyl-2-hydroxymuconate isomerase Function and homology information Function and homology information | |||||||||
| Biological species | synthetic construct (others) /  Pseudomonas aeruginosa PAO1 (bacteria) / synthetic (others) / Pseudomonas aeruginosa PAO1 (bacteria) / synthetic (others) /  Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria) Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.49 Å | |||||||||
 Authors Authors | Gonen S / Liu Y / Yeates TO / Gonen T | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2018 Journal: Proc Natl Acad Sci U S A / Year: 2018Title: Near-atomic cryo-EM imaging of a small protein displayed on a designed scaffolding system. Authors: Yuxi Liu / Shane Gonen / Tamir Gonen / Todd O Yeates /  Abstract: Current single-particle cryo-electron microscopy (cryo-EM) techniques can produce images of large protein assemblies and macromolecular complexes at atomic level detail without the need for crystal ...Current single-particle cryo-electron microscopy (cryo-EM) techniques can produce images of large protein assemblies and macromolecular complexes at atomic level detail without the need for crystal growth. However, proteins of smaller size, typical of those found throughout the cell, are not presently amenable to detailed structural elucidation by cryo-EM. Here we use protein design to create a modular, symmetrical scaffolding system to make protein molecules of typical size suitable for cryo-EM. Using a rigid continuous alpha helical linker, we connect a small 17-kDa protein (DARPin) to a protein subunit that was designed to self-assemble into a cage with cubic symmetry. We show that the resulting construct is amenable to structural analysis by single-particle cryo-EM, allowing us to identify and solve the structure of the attached small protein at near-atomic detail, ranging from 3.5- to 5-Å resolution. The result demonstrates that proteins considerably smaller than the theoretical limit of 50 kDa for cryo-EM can be visualized clearly when arrayed in a rigid fashion on a symmetric designed protein scaffold. Furthermore, because the amino acid sequence of a DARPin can be chosen to confer tight binding to various other protein or nucleic acid molecules, the system provides a future route for imaging diverse macromolecules, potentially broadening the application of cryo-EM to proteins of typical size in the cell. | |||||||||
| History |
|
- Structure visualization
Structure visualization
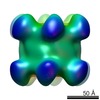
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7437.map.gz emd_7437.map.gz | 32.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7437-v30.xml emd-7437-v30.xml emd-7437.xml emd-7437.xml | 11.8 KB 11.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7437.png emd_7437.png | 163.9 KB | ||
| Filedesc metadata |  emd-7437.cif.gz emd-7437.cif.gz | 5.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7437 http://ftp.pdbj.org/pub/emdb/structures/EMD-7437 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7437 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7437 | HTTPS FTP |
-Related structure data
| Related structure data |  6c9kMC  7403C  7436C  6c9iC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7437.map.gz / Format: CCP4 / Size: 190.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7437.map.gz / Format: CCP4 / Size: 190.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Single-Particle reconstruction of DARP14 - A designed protein scaffold displaying ~17kDa DARPin proteins | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.655 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
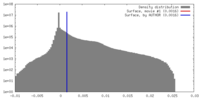
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : DARP14
| Entire | Name: DARP14 |
|---|---|
| Components |
|
-Supramolecule #1: DARP14
| Supramolecule | Name: DARP14 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Supramolecule #2: DARP14 - Subunit A with DARPin
| Supramolecule | Name: DARP14 - Subunit A with DARPin / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Supramolecule #3: DARP14 - Subunit B
| Supramolecule | Name: DARP14 - Subunit B / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Pseudomonas aeruginosa PAO1 (bacteria) Pseudomonas aeruginosa PAO1 (bacteria) |
-Macromolecule #1: DARP14 - Subunit A with DARPin
| Macromolecule | Name: DARP14 - Subunit A with DARPin / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic (others) |
| Molecular weight | Theoretical: 35.018965 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRITTKVGDK GSTRLFGGEE VWKDSPIIEA NGTLDELTSF IGEAKHYVDE EMKGILEEIQ NDIYKIMGEI GSKGKIEGIS EERIAWLLK LILRYMEMVN LKSFVLPGGT LESAKLDVCR TIARRALRKV LTVTREFGIG AEAAAYLLAL SDLLFLLARV I EIELGKKL ...String: MRITTKVGDK GSTRLFGGEE VWKDSPIIEA NGTLDELTSF IGEAKHYVDE EMKGILEEIQ NDIYKIMGEI GSKGKIEGIS EERIAWLLK LILRYMEMVN LKSFVLPGGT LESAKLDVCR TIARRALRKV LTVTREFGIG AEAAAYLLAL SDLLFLLARV I EIELGKKL LEAARAGQDD EVRILMANGA DVNAHDDQGS TPLHLAAWIG HPEIVEVLLK HGADVNARDT DGWTPLHLAA DN GHLEIVE VLLKYGADVN AQDAYGLTPL HLAADRGHLE IVEVLLKHGA DVNAQDKFGK TAFDISIDNG NEDLAEILQK LN |
-Macromolecule #2: DARP14 - Subunit B
| Macromolecule | Name: DARP14 - Subunit B / type: protein_or_peptide / ID: 2 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria) Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria)Strain: ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1 |
| Molecular weight | Theoretical: 14.346274 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPHLVIEATA NLRLETSPGE LLEQANKALF ASGQFGEADI KSRFVTLEAY RQGTAAVERA YLHACLSILD GRDIATRTLL GASLCAVLA EAVAGGGEEG VQVSVEVREM ERLSYAKRVV ARQRLEHHHH HH UniProtKB: 5-carboxymethyl-2-hydroxymuconate isomerase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Applied symmetry - Point group: T (tetrahedral) / Resolution.type: BY AUTHOR / Resolution: 3.49 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 183753 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)