[English] 日本語
 Yorodumi
Yorodumi- EMDB-6979: E. coli 50S subunit bound HflX protein in presence of ATP (AMP-PN... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6979 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli 50S subunit bound HflX protein in presence of ATP (AMP-PNP) and GTP (GMP-PNP) analogs. | |||||||||
 Map data Map data | E. coli 50S bound HflX protein in presence of ATP (AMP-PNP) and GTP (GMP-PNP) analogs. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATPase / RNA helicase / Heat stress / RIBOSOME | |||||||||
| Function / homology |  Function and homology information Function and homology informationribosome disassembly / guanosine tetraphosphate binding / ribosomal large subunit binding / rescue of stalled cytosolic ribosome / ribosome binding / response to heat / rRNA binding / GTPase activity / GTP binding / ATP hydrolysis activity ...ribosome disassembly / guanosine tetraphosphate binding / ribosomal large subunit binding / rescue of stalled cytosolic ribosome / ribosome binding / response to heat / rRNA binding / GTPase activity / GTP binding / ATP hydrolysis activity / ATP binding / metal ion binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
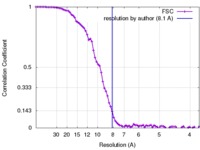
| Method | single particle reconstruction / cryo EM / Resolution: 8.1 Å | |||||||||
 Authors Authors | Dey S | |||||||||
 Citation Citation |  Journal: J Cell Biol / Year: 2018 Journal: J Cell Biol / Year: 2018Title: The universally conserved GTPase HflX is an RNA helicase that restores heat-damaged ribosomes. Authors: Sandip Dey / Chiranjit Biswas / Jayati Sengupta /  Abstract: The ribosome-associated GTPase HflX acts as an antiassociation factor upon binding to the 50S ribosomal subunit during heat stress in Although HflX is recognized as a guanosine triphosphatase, ...The ribosome-associated GTPase HflX acts as an antiassociation factor upon binding to the 50S ribosomal subunit during heat stress in Although HflX is recognized as a guanosine triphosphatase, several studies have shown that the N-terminal domain 1 of HflX is capable of hydrolyzing adenosine triphosphate (ATP), but the functional role of its adenosine triphosphatase (ATPase) activity remains unknown. We demonstrate that HflX possesses ATP-dependent RNA helicase activity and is capable of unwinding large subunit ribosomal RNA. A cryo-electron microscopy structure of the 50S-HflX complex in the presence of nonhydrolyzable analogues of ATP and guanosine triphosphate hints at a mode of action for the RNA helicase and suggests the linker helical domain may have a determinant role in RNA unwinding. Heat stress results in inactivation of the ribosome, and we show that HflX can restore heat-damaged ribosomes and improve cell survival. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6979.map.gz emd_6979.map.gz | 33.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6979-v30.xml emd-6979-v30.xml emd-6979.xml emd-6979.xml | 15.9 KB 15.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_6979_fsc.xml emd_6979_fsc.xml | 8.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_6979.png emd_6979.png | 278.3 KB | ||
| Filedesc metadata |  emd-6979.cif.gz emd-6979.cif.gz | 6.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6979 http://ftp.pdbj.org/pub/emdb/structures/EMD-6979 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6979 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6979 | HTTPS FTP |
-Related structure data
| Related structure data |  5zzmMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6979.map.gz / Format: CCP4 / Size: 35.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6979.map.gz / Format: CCP4 / Size: 35.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli 50S bound HflX protein in presence of ATP (AMP-PNP) and GTP (GMP-PNP) analogs. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.89 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : E. coli 50S bound HflX protein in presence of ATP (AMP-PNP) and G...
| Entire | Name: E. coli 50S bound HflX protein in presence of ATP (AMP-PNP) and GTP (GMP-PNP) analogs. |
|---|---|
| Components |
|
-Supramolecule #1: E. coli 50S bound HflX protein in presence of ATP (AMP-PNP) and G...
| Supramolecule | Name: E. coli 50S bound HflX protein in presence of ATP (AMP-PNP) and GTP (GMP-PNP) analogs. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: GTPase HflX
| Macromolecule | Name: GTPase HflX / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.392988 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFDRYDAGEQ AVLVHIYFTQ DKDMEDLQEF ESLVSSAGVE ALQVITGSRK APHPKYFVGE GKAVEIAEAV KATGASVVLF DHALSPAQE RNLERLCECR VIDRTGLILD IFAQRARTHE GKLQVELAQL RHLATRLVRG WTHLERQKGG IGLRGPGETQ L ETDRRLLR ...String: MFDRYDAGEQ AVLVHIYFTQ DKDMEDLQEF ESLVSSAGVE ALQVITGSRK APHPKYFVGE GKAVEIAEAV KATGASVVLF DHALSPAQE RNLERLCECR VIDRTGLILD IFAQRARTHE GKLQVELAQL RHLATRLVRG WTHLERQKGG IGLRGPGETQ L ETDRRLLR NRIVQIQSRL ERVEKQREQG RQSRIKADVP TVSLVGYTNA GKSTLFNRIT EARVYAADQL FATLDPTLRR ID VADVGET VLADTVGFIR HLPHDLVAAF KATLQETRQA TLLLHVIDAA DVRVQENIEA VNTVLEEIDA HEIPTLLVMN KID MLEDFE PRIDRDEENK PNRVWLSAQT GAGIPQLFQA LTERLSGEVA QHTLRLPPQE GRLRSRFYQL QAIEKEWMEE DGSV SLQVR MPIVDWRRLC KQEPALIDYL I UniProtKB: GTPase HflX |
-Macromolecule #2: 5S rRNA
| Macromolecule | Name: 5S rRNA / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 38.79009 KDa |
| Sequence | String: UGCCUGGCGG CCGUAGCGCG GUGGUCCCAC CUGACCCCAU GCCGAACUCA GAAGUGAAAC GCCGUAGCGC CGAUGGUAGU GUGGGGUCU CCCCAUGCGA GAGUAGGGAA CUGCCAGGCA U GENBANK: GENBANK: CP027586.1 |
-Macromolecule #3: 23S rRNA
| Macromolecule | Name: 23S rRNA / type: rna / ID: 3 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 941.306188 KDa |
| Sequence | String: GGUUAAGCGA CUAAGCGUAC ACGGUGGAUG CCCUGGCAGU CAGAGGCGAU GAAGGACGUG CUAAUCUGCG AUAAGCGUCG GUAAGGUGA UAUGAACCGU UAUAACCGGC GAUUUCCGAA UGGGGAAACC CAGUGUGUUU CGACACACUA UCAUUAACUG A AUCCAUAG ...String: GGUUAAGCGA CUAAGCGUAC ACGGUGGAUG CCCUGGCAGU CAGAGGCGAU GAAGGACGUG CUAAUCUGCG AUAAGCGUCG GUAAGGUGA UAUGAACCGU UAUAACCGGC GAUUUCCGAA UGGGGAAACC CAGUGUGUUU CGACACACUA UCAUUAACUG A AUCCAUAG GUUAAUGAGG CGAACCGGGG GAACUGAAAC AUCUAAGUAC CCCGAGGAAA AGAAAUCAAC CGAGAUUCCC CC AGUAGCG GCGAGCGAAC GGGGAGCAGC CCAGAGCCUG AAUCAGUGUG UGUGUUAGUG GAAGCGUCUG GAAAGGCGCG CGA UACAGG GUGACAGCCC CGUACACAAA AAUGCACAUG CUGUGAGCUC GAUGAGUAGG GCGGGACACG UGGUAUCCUG UCUG AAUAU GGGGGGACCA UCCUCCAAGG CUAAAUACUC CUGACUGACC GAUAGUGAAC CAGUACCGUG AGGGAAAGGC GAAAA GAAC CCCGGCGAGG GGAGUGAAAA AGAACCUGAA ACCGUGUACG UACAAGCAGU GGGAGCACGC UUAGGCGUGU GACUGC GUA CCUUUUGUAU AAUGGGUCAG CGACUUAUAU UCUGUAGCAA GGUUAACCGA AUAGGGGAGC CGAAGGGAAA CCGAGUC UU AACUGGGCGU UAAGUUGCAG GGUAUAGACC CGAAACCCGG UGAUCUAGCC AUGGGCAGGU UGAAGGUUGG GUAACACU A ACUGGAGGAC CGAACCGACU AAUGUUGAAA AAUUAGCGGA UGACUUGUGG CUGGGGGUGA AAGGCCAAUC AAACCGGGA GAUAGCUGGU UCUCCCCGAA AGCUAUUUAG GUAGCGCCUC GUGAAUUCAU CUCCGGGGGU AGAGCACUGU UUCGGCAAGG GGGUCAUCC CGACUUACCA ACCCGAUGCA AACUGCGAAU ACCGGAGAAU GUUAUCACGG GAGACACACG GCGGGUGCUA A CGUCCGUC GUGAAGAGGG AAACAACCCA GACCGCCAGC UAAGGUCCCA AAGUCAUGGU UAAGUGGGAA ACGAUGUGGG AA GGCCCAG ACAGCCAGGA UGUUGGCUUA GAAGCAGCCA UCAUUUAAAG AAAGCGUAAU AGCUCACUGG UCGAGUCGGC CUG CGCGGA AGAUGUAACG GGGCUAAACC AUGCACCGAA GCUGCGGCAG CGACGCUUAU GCGUUGUUGG GUAGGGGAGC GUUC UGUAA GCCUGCGAAG GUGUGCUGUG AGGCAUGCUG GAGGUAUCAG AAGUGCGAAU GCUGACAUAA GUAACGAUAA AGCGG GUGA AAAGCCCGCU CGCCGGAAGA CCAAGGGUUC CUGUCCAACG UUAAUCGGGG CAGGGUGAGU CGACCCCUAA GGCGAG GCC GAAAGGCGUA GUCGAUGGGA AACAGGUUAA UAUUCCUGUA CUUGGUGUUA CUGCGAAGGG GGGACGGAGA AGGCUAU GU UGGCCGGGCG ACGGUUGUCC CGGUUUAAGC GUGUAGGCUG GUUUUCCAGG CAAAUCCGGA AAAUCAAGGC UGAGGCGU G AUGACGAGGC ACUACGGUGC UGAAGCAACA AAUGCCCUGC UUCCAGGAAA AGCCUCUAAG CAUCAGGUAA CAUCAAAUC GUACCCCAAA CCGACACAGG UGGUCAGGUA GAGAAUACCA AGGCGCUUGA GAGAACUCGG GUGAAGGAAC UAGGCAAAAU GGUGCCGUA ACUUCGGGAG AAGGCACGCU GAUAUGUAGG UGAGGUCCCU CGCGGAUGGA GCUGAAAUCA GUCGAAGAUA C CAGCUGGC UGCAACUGUU UAUUAAAAAC ACAGCACUGU GCAAACACGA AAGUGGACGU AUACGGUGUG ACGCCUGCCC GG UGCCGGA AGGUUAAUUG AUGGGGUUAG CGCAAGCGAA GCUCUUGAUC GAAGCCCCGG UAAACGGCGG CCGUAACUAU AAC GGUCCU AAGGUAGCGA AAUUCCUUGU CGGGUAAGUU CCGACCUGCA CGAAUGGCGU AAUGAUGGCC AGGCUGUCUC CACC CGAGA CUCAGUGAAA UUGAACUCGC UGUGAAGAUG CAGUGUACCC GCGGCAAGAC GGAAAGACCC CGUGAACCUU UACUA UAGC UUGACACUGA ACAUUGAGCC UUGAUGUGUA GGAUAGGUGG GAGGCUUUGA AGUGUGGACG CCAGUCUGCA UGGAGC CGA CCUUGAAAUA CCACCCUUUA AUGUUUGAUG UUCUAACGUU GACCCGUAAU CCGGGUUGCG GACAGUGUCU GGUGGGU AG UUUGACUGGG GCGGUCUCCU CCUAAAGAGU AACGGAGGAG CACGAAGGUU GGCUAAUCCU GGUCGGACAU CAGGAGGU U AGUGCAAUGG CAUAAGCCAG CUUGACUGCG AGCGUGACGG CGCGAGCAGG UGCGAAAGCA GGUCAUAGUG AUCCGGUGG UUCUGAAUGG AAGGGCCAUC GCUCAACGGA UAAAAGGUAC UCCGGGGAUA ACAGGCUGAU ACCGCCCAAG AGUUCAUAUC GACGGCGGU GUUUGGCACC UCGAUGUCGG CUCAUCACAU CCUGGGGCUG AAGUAGGUCC CAAGGGUAUG GCUGUUCGCC A UUUAAAGU GGUACGCGAG CUGGGUUUAG AACGUCGUGA GACAGUUCGG UCCCUAUCUG CCGUGGGCGC UGGAGAACUG AG GGGGGCU GCUCCUAGUA CGAGAGGACC GGAGUGGACG CAUCACUGGU GUUCGGGUUG UCAUGCCAAU GGCACUGCCC GGU AGCUAA AUGCGGAAGA GAUAAGUGCU GAAAGCAUCU AAGCACGAAA CUUGCCCCGA GAUGAGUUCU CCCUGACCCU UUAA GGGUC CUGAAGGAAC GUUGAAGACG ACGACGUUGA UAGGCCGGGU GUGUAAGCGC AGCGAUGCGU UGAGCUAACC GGUAC UAAU GAACCGUGAG GCUUAACCU GENBANK: GENBANK: CP016018.1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: LACEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: FEI EAGLE (4k x 4k) / Number grids imaged: 4 / Average exposure time: 2.0 sec. / Average electron dose: 20.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DIFFRACTION |
| Sample stage | Specimen holder model: GATAN 910 MULTI-SPECIMEN SINGLE TILT CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-5zzm: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)