+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Sarbecovirus BtKY72 Spike Trimer in a Locked Conformation | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | spike protein / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / host cell plasma membrane / virion membrane ...host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / host cell plasma membrane / virion membrane / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  BtKY72 (virus) / BtKY72 (virus) /  Kenya bat coronavirus BtKY72 Kenya bat coronavirus BtKY72 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Wang J / Xiong X | |||||||||
| Funding support | 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2025 Journal: Sci Adv / Year: 2025Title: SARS-related coronavirus S-protein structures reveal synergistic RBM interactions underpinning high-affinity human ACE2 binding. Authors: Jingjing Wang / Yong Ma / Zimu Li / Hang Yuan / Banghui Liu / Zexuan Li / Mengzhen Su / Gul Habib / Yutong Liu / Lutang Fu / Peiyi Wang / Mei Li / Jun He / Jing Chen / Peng Zhou / Zhengli ...Authors: Jingjing Wang / Yong Ma / Zimu Li / Hang Yuan / Banghui Liu / Zexuan Li / Mengzhen Su / Gul Habib / Yutong Liu / Lutang Fu / Peiyi Wang / Mei Li / Jun He / Jing Chen / Peng Zhou / Zhengli Shi / Xinwen Chen / Xiaoli Xiong /  Abstract: High-affinity and specific binding toward the human angiotensin-converting enzyme 2 (hACE2) receptor by severe acute respiratory syndrome coronavirus (SARS)-related coronaviruses (SARSr-CoVs) remains ...High-affinity and specific binding toward the human angiotensin-converting enzyme 2 (hACE2) receptor by severe acute respiratory syndrome coronavirus (SARS)-related coronaviruses (SARSr-CoVs) remains incompletely understood. We report cryo-electron microscopy structures of eight different S-proteins from SARSr-CoVs found across Asia, Europe, and Africa. These S-proteins all adopt tightly packed, locked, prefusion conformations. These structures enable the classification of SARSr-CoV S-proteins into three types, based on their receptor-binding motif (RBM) structures and ACE2 binding characteristics. Type-2 S-proteins often preferentially bind bat ACE2 (bACE2) over hACE2. We report a structure of a type-2 BtKY72-RBD in complex with bACE2 to understand ACE2 specificity. Structure-guided mutagenesis of BtKY72-RBD reveals that multiple synergistic mutations in four different regions of RBM are required to achieve high-affinity hACE2 binding. Similar RBM changes can also confer hACE2 binding to another type-2 BM48-31 S-protein, which is primarily non-ACE2 binding. These results provide an understanding of how high-affinity hACE2 binding may be acquired by SARSr-CoV S-proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_60551.map.gz emd_60551.map.gz | 40.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-60551-v30.xml emd-60551-v30.xml emd-60551.xml emd-60551.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_60551.png emd_60551.png | 52.2 KB | ||
| Filedesc metadata |  emd-60551.cif.gz emd-60551.cif.gz | 7.5 KB | ||
| Others |  emd_60551_half_map_1.map.gz emd_60551_half_map_1.map.gz emd_60551_half_map_2.map.gz emd_60551_half_map_2.map.gz | 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-60551 http://ftp.pdbj.org/pub/emdb/structures/EMD-60551 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60551 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60551 | HTTPS FTP |
-Validation report
| Summary document |  emd_60551_validation.pdf.gz emd_60551_validation.pdf.gz | 821.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_60551_full_validation.pdf.gz emd_60551_full_validation.pdf.gz | 820.8 KB | Display | |
| Data in XML |  emd_60551_validation.xml.gz emd_60551_validation.xml.gz | 12.2 KB | Display | |
| Data in CIF |  emd_60551_validation.cif.gz emd_60551_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60551 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60551 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60551 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60551 | HTTPS FTP |
-Related structure data
| Related structure data |  8zy0MC  8zy1C  8zy2C  8zy3C  8zy4C  8zy5C  8zy6C  8zy7C  8zy9C  8zyaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_60551.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_60551.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.095 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_60551_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_60551_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : BtKY72/Rhinolophus sp./Kenya/2007
| Entire | Name: BtKY72/Rhinolophus sp./Kenya/2007 |
|---|---|
| Components |
|
-Supramolecule #1: BtKY72/Rhinolophus sp./Kenya/2007
| Supramolecule | Name: BtKY72/Rhinolophus sp./Kenya/2007 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: the spike trimer |
|---|---|
| Source (natural) | Organism:  BtKY72 (virus) BtKY72 (virus) |
| Molecular weight | Theoretical: 420 KDa |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 Details: delete residues 1194-1257.Sequence reference for source organism Kenya bat coronavirus BtKY72 is not available in UniProt at the time of biocuration. Current sequence reference is from UniProt id A0A3Q8AKM0. Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Kenya bat coronavirus BtKY72 Kenya bat coronavirus BtKY72 |
| Molecular weight | Theoretical: 132.339625 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKFFILLSLL SFTTAQEGCG ILSNKSNPAL TQYFSSRRGF YYFDDTFRSS VRVLTTGYFL PFNSNLTGYS SRNSVTGRLI QFDNPNIPF KDGLYFAATE RSNVIRGWIF GSTLDNTTQS AVLFNNGTHI VINVCNFYFC QDPMLAVANG SHFKSWVFLN A TNCTYNRV ...String: MKFFILLSLL SFTTAQEGCG ILSNKSNPAL TQYFSSRRGF YYFDDTFRSS VRVLTTGYFL PFNSNLTGYS SRNSVTGRLI QFDNPNIPF KDGLYFAATE RSNVIRGWIF GSTLDNTTQS AVLFNNGTHI VINVCNFYFC QDPMLAVANG SHFKSWVFLN A TNCTYNRV HGFEIDPSPN TGSFIHLREH VFRNVDGFLY VYHNYERVDV YDNFPQGFSV LKPIFKLPFG LNITQFKVIM TL FSPTTSS FNADASVYFV GHLKPLTMLA EFDENGTITD AVDCSQDPLS ELKCTTKSLT VEKGIYQTSN FRVSPSTEVV RFP NITNLC PFGQVFNASN FPSVYAWERL RISDCVADYA VLYNSSSSFS TFKCYGVSPT KLNDLCFSSV YADYFVVKGD DVRQ IAPAQ TGVIADYNYK LPDDFTGCVL AWNTNSVDSK SGNNFYYRLF RHGKIKPYER DISNVLYNSA GGTCSSISQL GCYEP LKSY GFTPTVGVGY QPYRVVVLSF ELLNAPATVC GPKKSTELVK NKCVNFNFNG LTGTGVLTSS TKKFQPFQQF GRDVSD FTD SVRDPKTFEI LDISPCSYGG VSVITPGTNT SKAVAVLYQD VNCTDVPTMI HVEQVSSDWR VYAFNSYGNM FQTQAGC LV GAIYENTTYE CDIPIGAGIC AKFGSDKIRM GQESIVAYTM SIGEDQSIAY SNNIIAIPTN FSISVTTEVL PVSMTKTS V DCNMYICGDS TECSNLLLQY GSFCTQLNRA LSGIAVEQDR NTRDVFAQTK SIYKTPNIKD FGGFNFSQIL PDPKKLSYR SFIEDLLYNK VTLSDPGFMK QYGDCLGGIN ARDLICAQKF NGLTVLPPLL TDDMIAAYTA ALISGTATAG YTFGAGAALQ IPFAMQMAY RFNGIGVTQN VLYENQKQIA NQFNNAISKI QDSLTTTSAA LGKLQDVINQ NAVALNTLVK QLSSNFGAIS S VLNDILSR LDKVEAEVQI DRLITGRLQS LQTYVTQQLI RAAEIRASAN LAATKMSECV LGQSKRVDFC GKGYHLMSFP QA APHGVVF LHVTYVPSQQ QNFTTAPAIC HNGKAYFPRE GVFVMNGTHW FITQRNFYSP QVITTDNTFE SGSCDVVIGI INN TVYDPL QPELESFKQE LDKYFKNHTS PDVDFGDISG INASVVDIKK EIAHLNEIAK NLNESLIDLQ ELGKYEQ UniProtKB: Spike glycoprotein |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 51 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #6: BILIVERDINE IX ALPHA
| Macromolecule | Name: BILIVERDINE IX ALPHA / type: ligand / ID: 6 / Number of copies: 3 / Formula: BLA |
|---|---|
| Molecular weight | Theoretical: 582.646 Da |
| Chemical component information | 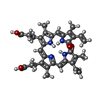 ChemComp-BLA: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
Details: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





































