[English] 日本語
 Yorodumi
Yorodumi- EMDB-60269: Cryo-EM structure of W89F mutated Glutamate dehydrogenase from Th... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of W89F mutated Glutamate dehydrogenase from Thermococcus profundus in complex with NADP and GLU in the steady stage of reaction | ||||||||||||||||||||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | Oxidoreductase / Complex / NADP / Glutamate / Mutant | ||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationglutamate dehydrogenase [NAD(P)+] / glutamate dehydrogenase (NAD+) activity / glutamate dehydrogenase (NADP+) activity / L-glutamate catabolic process Similarity search - Function | ||||||||||||||||||||||||||||||||||||||||||
| Biological species |   Thermococcus profundus (archaea) Thermococcus profundus (archaea) | ||||||||||||||||||||||||||||||||||||||||||
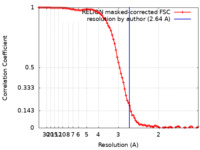
| Method | single particle reconstruction / cryo EM / Resolution: 2.64 Å | ||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Wakabayashi T / Nakasako M | ||||||||||||||||||||||||||||||||||||||||||
| Funding support |  Japan, 13 items Japan, 13 items
| ||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: FEBS J / Year: 2025 Journal: FEBS J / Year: 2025Title: CryoEM and crystal structure analyses reveal the indirect role played by Trp89 in glutamate dehydrogenase enzymatic reactions. Authors: Taiki Wakabayashi / Yuka Matsui / Masayoshi Nakasako /  Abstract: Glutamate dehydrogenase from Thermococcus profundus is a homo-hexameric enzyme that catalyzes the reversible deamination of glutamate to 2-oxoglutarate in the presence of a cofactor. In each subunit, ...Glutamate dehydrogenase from Thermococcus profundus is a homo-hexameric enzyme that catalyzes the reversible deamination of glutamate to 2-oxoglutarate in the presence of a cofactor. In each subunit, a large active-site cleft is formed between the two functional domains, one of which displays motion to open and close the cleft. Trp89 in the cleft displays two sidechain conformers in the open cleft and a single conformer in the closed cleft. To reveal the role of the Trp89 sidechain in the domain motion, we mutated Trp89 to phenylalanine. Despite the Trp89 sidechain being located away from the reaction center, the catalytic constant decreased to 1/38-fold of that of the wild-type without a fatal reduction of the affinities to the cofactor and ligand molecules. To understand the molecular mechanism underlying this reduction, we determined the crystal structure in the unliganded state and the metastable conformations appearing in the steady stage of the reaction using cryo-electron microscopy (cryoEM). The four identified metastable conformations were similar to the three conformations observed in the wild-type, but their populations were different from those of the wild-type. In addition, a conformation with a completely closed active-site cleft necessary for the reaction to proceed was quite rare. The crystal structure and the four metastable conformations suggested that the reduction in the catalytic constant could be attributed to changes in the interactions between Gln13 and the 89th side chains, preventing the closing domain motion. | ||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_60269.map.gz emd_60269.map.gz | 14.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-60269-v30.xml emd-60269-v30.xml emd-60269.xml emd-60269.xml | 24.1 KB 24.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_60269_fsc.xml emd_60269_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_60269.png emd_60269.png | 90.6 KB | ||
| Masks |  emd_60269_msk_1.map emd_60269_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-60269.cif.gz emd-60269.cif.gz | 7.2 KB | ||
| Others |  emd_60269_half_map_1.map.gz emd_60269_half_map_1.map.gz emd_60269_half_map_2.map.gz emd_60269_half_map_2.map.gz | 49.8 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-60269 http://ftp.pdbj.org/pub/emdb/structures/EMD-60269 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60269 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60269 | HTTPS FTP |
-Validation report
| Summary document |  emd_60269_validation.pdf.gz emd_60269_validation.pdf.gz | 833.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_60269_full_validation.pdf.gz emd_60269_full_validation.pdf.gz | 832.9 KB | Display | |
| Data in XML |  emd_60269_validation.xml.gz emd_60269_validation.xml.gz | 16.2 KB | Display | |
| Data in CIF |  emd_60269_validation.cif.gz emd_60269_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60269 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60269 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60269 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-60269 | HTTPS FTP |
-Related structure data
| Related structure data |  8zneMC  8zmuC  8znbC  8zncC  8zndC  8zngC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_60269.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_60269.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.752 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_60269_msk_1.map emd_60269_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_60269_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_60269_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Hexamer of W89F mutated glutamate dehydrogenase
| Entire | Name: Hexamer of W89F mutated glutamate dehydrogenase |
|---|---|
| Components |
|
-Supramolecule #1: Hexamer of W89F mutated glutamate dehydrogenase
| Supramolecule | Name: Hexamer of W89F mutated glutamate dehydrogenase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Thermococcus profundus (archaea) Thermococcus profundus (archaea) |
| Molecular weight | Theoretical: 280 KDa |
-Macromolecule #1: Glutamate dehydrogenase
| Macromolecule | Name: Glutamate dehydrogenase / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: glutamate dehydrogenase [NAD(P)+] |
|---|---|
| Source (natural) | Organism:   Thermococcus profundus (archaea) Thermococcus profundus (archaea) |
| Molecular weight | Theoretical: 46.360004 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: IDPFEMAVKQ LERAAQYMDI SEEALEWLKK PMRIVEVSVP IEMDDGSVKV FTGFRVQHNW ARGPTKGGIR WHPAETLSTV KALATFMTW KVAVVDLPYG GGKGGIIVNP KELSEREQER LARAYIRAVY DVIGPWTDIP APDVYTNPKI MGWMMDEYET I MRRKGPAF ...String: IDPFEMAVKQ LERAAQYMDI SEEALEWLKK PMRIVEVSVP IEMDDGSVKV FTGFRVQHNW ARGPTKGGIR WHPAETLSTV KALATFMTW KVAVVDLPYG GGKGGIIVNP KELSEREQER LARAYIRAVY DVIGPWTDIP APDVYTNPKI MGWMMDEYET I MRRKGPAF GVITGKPLSI GGSLGRGTAT AQGAIFTIRE AAKALGIDLK GKKIAVQGYG NAGYYTAKLA KEQLGMTVVA VS DSRGGIY NPDGLDPDEV LKWKREHGSV KDFPGATNIT NEELLELEVD VLAPAAIEEV ITEKNADNIK AKIVAEVANG PVT PEADDI LREKGILQIP DFLCNAGGVT VSYFEWVQNI NGYYWTEEEV REKLDKKMTK AFWEVYNTHK DKNIHMRDAA YVVA VSRVY QAMKDRGWVK K UniProtKB: Glutamate dehydrogenase |
-Macromolecule #2: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE
| Macromolecule | Name: NADP NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE / type: ligand / ID: 2 / Number of copies: 1 / Formula: NAP |
|---|---|
| Molecular weight | Theoretical: 743.405 Da |
| Chemical component information |  ChemComp-NAP: |
-Macromolecule #3: GLUTAMIC ACID
| Macromolecule | Name: GLUTAMIC ACID / type: ligand / ID: 3 / Number of copies: 1 / Formula: GLU |
|---|---|
| Molecular weight | Theoretical: 147.129 Da |
| Chemical component information |  ChemComp-GLU: |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 9 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 9.0 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 0.5 mM NADP, 100 mM Glutamate in 5 mM Tris-HCl at pH7.5. | ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.02 kPa Details: Both sides of the grid were glow-discharged for 45 s at 20 mA and 20 Pa. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: The sample solution was flash-frozen 2-h after mixing the protein solution and buffer solution.. | ||||||||||||
| Details | 8.96 mg/mL GDH W89F |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 7651 / Average exposure time: 2.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 5.0 µm / Nominal defocus min: 0.35000000000000003 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)