[English] 日本語
 Yorodumi
Yorodumi- EMDB-5763: Electron cryo-microscopy of two-stranded TubZ filament from B. th... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5763 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Electron cryo-microscopy of two-stranded TubZ filament from B. thuringiensis | |||||||||
 Map data Map data | Helical Reconstruction of two-stranded TubZ-Bt bound to GTPgammaS | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | plasmid segregation / tubulin family / bacterial cytoskeleton | |||||||||
| Function / homology |  Function and homology information Function and homology informationplasmid partitioning / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / GTPase activity / GTP binding / metal ion binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 10.8 Å | |||||||||
 Authors Authors | Montabana EA / Agard DA | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2014 Journal: Proc Natl Acad Sci U S A / Year: 2014Title: Bacterial tubulin TubZ-Bt transitions between a two-stranded intermediate and a four-stranded filament upon GTP hydrolysis. Authors: Elizabeth A Montabana / David A Agard /  Abstract: Cytoskeletal filaments form diverse superstructures that are highly adapted for specific functions. The recently discovered TubZ subfamily of tubulins is involved in type III plasmid partitioning ...Cytoskeletal filaments form diverse superstructures that are highly adapted for specific functions. The recently discovered TubZ subfamily of tubulins is involved in type III plasmid partitioning systems, facilitating faithful segregation of low copy-number plasmids during bacterial cell division. One such protein, TubZ-Bt, is found on the large pBtoxis plasmid in Bacillus thuringiensis, and interacts via its extended C terminus with a DNA adaptor protein TubR. Here, we use cryo-electron microscopy to determine the structure of TubZ-Bt filaments and light scattering to explore their mechanism of polymerization. Surprisingly, we find that the helical filament architecture is remarkably sensitive to nucleotide state, changing from two-stranded to four-stranded depending on the ability of TubZ-Bt to hydrolyze GTP. We present pseudoatomic models of both the two- and four-protofilament forms based on cryo-electron microscopy reconstructions (10.8 Å and 6.9 Å, respectively) of filaments formed under different nucleotide states. These data lead to a model in which the two-stranded filament is a necessary intermediate along the pathway to formation of the four-stranded filament. Such nucleotide-directed structural polymorphism is to our knowledge an unprecedented mechanism for the formation of polar filaments. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5763.map.gz emd_5763.map.gz | 10.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5763-v30.xml emd-5763-v30.xml emd-5763.xml emd-5763.xml | 10.8 KB 10.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5763.jpg emd_5763.jpg | 37 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5763 http://ftp.pdbj.org/pub/emdb/structures/EMD-5763 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5763 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5763 | HTTPS FTP |
-Validation report
| Summary document |  emd_5763_validation.pdf.gz emd_5763_validation.pdf.gz | 349.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5763_full_validation.pdf.gz emd_5763_full_validation.pdf.gz | 348.8 KB | Display | |
| Data in XML |  emd_5763_validation.xml.gz emd_5763_validation.xml.gz | 5.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5763 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5763 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5763 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5763 | HTTPS FTP |
-Related structure data
| Related structure data |  3j4tMC  5762C  3j4sC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_5763.map.gz / Format: CCP4 / Size: 39.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5763.map.gz / Format: CCP4 / Size: 39.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Helical Reconstruction of two-stranded TubZ-Bt bound to GTPgammaS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.203 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
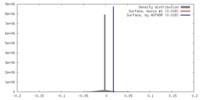
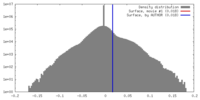
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : N-terminally tagged full length TubZ from pBtoxis in Bacillus thu...
| Entire | Name: N-terminally tagged full length TubZ from pBtoxis in Bacillus thuringiensis |
|---|---|
| Components |
|
-Supramolecule #1000: N-terminally tagged full length TubZ from pBtoxis in Bacillus thu...
| Supramolecule | Name: N-terminally tagged full length TubZ from pBtoxis in Bacillus thuringiensis type: sample / ID: 1000 Oligomeric state: Helical filament with two protofilament strands Number unique components: 1 |
|---|
-Macromolecule #1: ftsZ/tubulin-related protein
| Macromolecule | Name: ftsZ/tubulin-related protein / type: protein_or_peptide / ID: 1 / Name.synonym: TubZ-Bt, TubZ / Oligomeric state: helical two-stranded filament / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 54.3729 KDa |
| Recombinant expression | Organism:  |
| Sequence | UniProtKB: Tubulin-like protein TubZ / InterPro: Tubulin/FtsZ, GTPase domain |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.7 Details: 100 mM potassium acetate, 5 mM magnesium acetate, 50 mM HEPES |
|---|---|
| Grid | Details: 400 mesh copper grid with holey carbon support, glow discharged |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK III Timed resolved state: TubZ was preheated at 37 degrees and polymerization was initiated with saturating GTPgammaS. TubZ was then incubated for 30 seconds at room temperature before sample application and plunge-freezing. Method: Blot 4.5 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Alignment procedure | Legacy - Astigmatism: Astigmatism corrected at 250,000 times magnification |
| Date | May 26, 2010 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F816 (8k x 8k) / Number real images: 348 / Average electron dose: 20 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.2 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 62000 |
| Sample stage | Specimen holder model: OTHER |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | The particles were aligned using IHRSR. The azimuthal angle refined to 191.84151 degrees and the rise to 22.04811 Angstroms with a right-handed helix. |
|---|---|
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 22.04811 Å Applied symmetry - Helical parameters - Δ&Phi: 168.15849 ° Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 10.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: Spider Details: Final map has been low-pass filtered to 11 Angstrom and high-pass filtered to 35 Angstrom. A B-factor of -452 Angstroms was applied using the program bfactor. A cylindrical mask of radius ...Details: Final map has been low-pass filtered to 11 Angstrom and high-pass filtered to 35 Angstrom. A B-factor of -452 Angstroms was applied using the program bfactor. A cylindrical mask of radius ~78 Angstrom has been applied. |
| CTF correction | Details: Whole micrograph |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)