+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-5681 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Protein-Ligand Interactions by Electron Microscopy: a Disaccharide receptor analog complex of Adeno-Associated Virus | |||||||||
 マップデータ マップデータ | Reconstruction of Adeno-Associated Virus DJ | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Adeno-Associated Virus / contrast transfer function / heparin-binding domain / heparan sulfate proteoglycan / Virus-like particle | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報symbiont entry into host cell via permeabilization of host membrane / host cell nucleolus / T=1 icosahedral viral capsid / clathrin-dependent endocytosis of virus by host cell / virion attachment to host cell / structural molecule activity 類似検索 - 分子機能 | |||||||||
| 生物種 |   Adeno-associated virus (アデノ随伴ウイルス) Adeno-associated virus (アデノ随伴ウイルス) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 4.8 Å | |||||||||
 データ登録者 データ登録者 | Xie Q / Spilman M / Meyer N / Lerch T / Stagg S / Chapman M | |||||||||
 引用 引用 |  ジャーナル: J Struct Biol / 年: 2013 ジャーナル: J Struct Biol / 年: 2013タイトル: Electron microscopy analysis of a disaccharide analog complex reveals receptor interactions of adeno-associated virus. 著者: Qing Xie / Michael Spilman / Nancy L Meyer / Thomas F Lerch / Scott M Stagg / Michael S Chapman /  要旨: Mechanistic studies of macromolecular complexes often feature X-ray structures of complexes with bound ligands. The attachment of adeno-associated virus (AAV) to cell surface glycosaminoglycans (GAGs) ...Mechanistic studies of macromolecular complexes often feature X-ray structures of complexes with bound ligands. The attachment of adeno-associated virus (AAV) to cell surface glycosaminoglycans (GAGs) is an example that has not proven amenable to crystallography, because the binding of GAG analogs disrupts lattice contacts. The interactions of AAV with GAGs are of interest in mediating the cell specificity of AAV-based gene therapy vectors. Previous electron microscopy led to differing conclusions on the exact binding site and the existence of large ligand-induced conformational changes in the virus. Conformational changes are expected during cell entry, but it has remained unclear whether the electron microscopy provided evidence of their induction by GAG-binding. Taking advantage of automated data collection, careful processing and new methods of structure refinement, the structure of AAV-DJ complexed with sucrose octasulfate is determined by electron microscopy difference map analysis to 4.8Å resolution. At this higher resolution, individual sulfate groups are discernible, providing a stereochemical validation of map interpretation, and highlighting interactions with two surface arginines that have been implicated in genetic studies. Conformational changes induced by the SOS are modest and limited to the loop most directly interacting with the ligand. While the resolution attainable will depend on sample order and other factors, there are an increasing number of macromolecular complexes that can be studied by cryo-electron microscopy at resolutions beyond 5Å, for which the approaches used here could be used to characterize the binding of inhibitors and other small molecule effectors when crystallography is not tractable. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_5681.map.gz emd_5681.map.gz | 68.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-5681-v30.xml emd-5681-v30.xml emd-5681.xml emd-5681.xml | 11.7 KB 11.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_5681_1.jpg emd_5681_1.jpg | 409.2 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5681 http://ftp.pdbj.org/pub/emdb/structures/EMD-5681 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5681 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5681 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_5681_validation.pdf.gz emd_5681_validation.pdf.gz | 404 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_5681_full_validation.pdf.gz emd_5681_full_validation.pdf.gz | 403.5 KB | 表示 | |
| XML形式データ |  emd_5681_validation.xml.gz emd_5681_validation.xml.gz | 6.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5681 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5681 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5681 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5681 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_5681.map.gz / 形式: CCP4 / 大きさ: 75 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_5681.map.gz / 形式: CCP4 / 大きさ: 75 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Reconstruction of Adeno-Associated Virus DJ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.3067 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
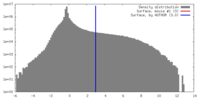
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Recombinant Adeno-Associated virus DJ with Sucrose Octasulfate
| 全体 | 名称: Recombinant Adeno-Associated virus DJ with Sucrose Octasulfate |
|---|---|
| 要素 |
|
-超分子 #1000: Recombinant Adeno-Associated virus DJ with Sucrose Octasulfate
| 超分子 | 名称: Recombinant Adeno-Associated virus DJ with Sucrose Octasulfate タイプ: sample / ID: 1000 集合状態: one virus subunit binds to one molecule of sucrose octasulfate Number unique components: 2 |
|---|---|
| 分子量 | 実験値: 3.7 MDa / 理論値: 3.7 MDa |
-超分子 #1: Adeno-associated virus
| 超分子 | 名称: Adeno-associated virus / タイプ: virus / ID: 1 / Name.synonym: Recombinant Adeno-Associated Virus DJ, AAVDJ / NCBI-ID: 272636 / 生物種: Adeno-associated virus / ウイルスタイプ: VIRUS-LIKE PARTICLE / ウイルス・単離状態: SEROTYPE / ウイルス・エンベロープ: No / ウイルス・中空状態: Yes Syn species name: Recombinant Adeno-Associated Virus DJ, AAVDJ Sci species serotype: DJ |
|---|---|
| 宿主 | 生物種:  Homo sapiens (ヒト) / 別称: VERTEBRATES Homo sapiens (ヒト) / 別称: VERTEBRATES |
| Host system | 生物種:  組換株: sf9 / 組換プラスミド: pAVDJ |
| 分子量 | 実験値: 3.7 MDa / 理論値: 3.7 MDa |
| ウイルス殻 | Shell ID: 1 / 直径: 275 Å / T番号(三角分割数): 1 |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1.5 mg/mL |
|---|---|
| 緩衝液 | pH: 6.8 / 詳細: 10 mM Tris, 125 mM NaCl, 1 mM MgCl2 |
| グリッド | 詳細: 400 mesh carbon-coated grids (C-flat) glow-discharged for 5 seconds (Gatan Model 950) |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / 装置: FEI VITROBOT MARK IV 手法: Both sides of the grid were blotted for 2.5 seconds. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 温度 | 平均: 94 K |
| アライメント法 | Legacy - 非点収差: Objective lens astigmatism was corrected at 120,000 times magnification |
| 日付 | 2013年4月15日 |
| 撮影 | カテゴリ: CCD フィルム・検出器のモデル: GATAN ULTRASCAN 4000 (4k x 4k) 実像数: 5207 / 平均電子線量: 15 e/Å2 |
| 電子線 | 加速電圧: 120 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 2.0 µm / 最小 デフォーカス(公称値): 0.8 µm / 倍率(公称値): 120000 |
| 試料ステージ | 試料ホルダー: Liquid nitrogen cooled 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 詳細 | Particles were processed semi-automatically using Appion for picking, contrast transfer function (CTF) estimation, and stack making. Particles were selected automatically using template matching. |
|---|---|
| CTF補正 | 詳細: CTF correction of each particle |
| 最終 再構成 | アルゴリズム: OTHER / 解像度のタイプ: BY AUTHOR / 解像度: 4.8 Å / 解像度の算出法: FSC 0.143 CUT-OFF / ソフトウェア - 名称: FREALIGN / 詳細: Final map was amplitude corrected using embfactor. / 使用した粒子像数: 45000 |
| 最終 角度割当 | 詳細: Frealign convention |
 ムービー
ムービー コントローラー
コントローラー



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)