[English] 日本語
 Yorodumi
Yorodumi- EMDB-5576: Electron Cryo-microscopy of Chikungunya VLP in complex with neutr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5576 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
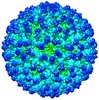
| Title | Electron Cryo-microscopy of Chikungunya VLP in complex with neutralizing antibody Fab m242 | |||||||||
 Map data Map data | Reconstruction of Chikungunya VLP with neutralizing antibody m242 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Alpha virus / Chikungunya VLP / neutralizing antibody m242 | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Chikungunya virus Chikungunya virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 15.6 Å | |||||||||
 Authors Authors | Sun S / Xiang Y / Rossmann MG | |||||||||
 Citation Citation |  Journal: Elife / Year: 2013 Journal: Elife / Year: 2013Title: Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization. Authors: Siyang Sun / Ye Xiang / Wataru Akahata / Heather Holdaway / Pankaj Pal / Xinzheng Zhang / Michael S Diamond / Gary J Nabel / Michael G Rossmann /  Abstract: A 5.3 Å resolution, cryo-electron microscopy (cryoEM) map of Chikungunya virus-like particles (VLPs) has been interpreted using the previously published crystal structure of the Chikungunya E1-E2 ...A 5.3 Å resolution, cryo-electron microscopy (cryoEM) map of Chikungunya virus-like particles (VLPs) has been interpreted using the previously published crystal structure of the Chikungunya E1-E2 glycoprotein heterodimer. The heterodimer structure was divided into domains to obtain a good fit to the cryoEM density. Differences in the T = 4 quasi-equivalent heterodimer components show their adaptation to different environments. The spikes on the icosahedral 3-fold axes and those in general positions are significantly different, possibly representing different phases during initial generation of fusogenic E1 trimers. CryoEM maps of neutralizing Fab fragments complexed with VLPs have been interpreted using the crystal structures of the Fab fragments and the VLP structure. Based on these analyses the CHK-152 antibody was shown to stabilize the viral surface, hindering the exposure of the fusion-loop, likely neutralizing infection by blocking fusion. The CHK-9, m10 and m242 antibodies surround the receptor-attachment site, probably inhibiting infection by blocking cell attachment. DOI:http://dx.doi.org/10.7554/eLife.00435.001. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5576.map.gz emd_5576.map.gz | 76 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5576-v30.xml emd-5576-v30.xml emd-5576.xml emd-5576.xml | 11.5 KB 11.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5576.png emd_5576.png | 360.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5576 http://ftp.pdbj.org/pub/emdb/structures/EMD-5576 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5576 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5576 | HTTPS FTP |
-Related structure data
| Related structure data |  3j2xMC  5577C  5578C  5579C  5580C  3j2wC  3j2yC  3j2zC  3j30C  4gq9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5576.map.gz / Format: CCP4 / Size: 238.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5576.map.gz / Format: CCP4 / Size: 238.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of Chikungunya VLP with neutralizing antibody m242 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.22 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Electron Cryo-microscopy of Chikungunya VLP in complex with neutr...
| Entire | Name: Electron Cryo-microscopy of Chikungunya VLP in complex with neutralizing antibody Fab m242 |
|---|---|
| Components |
|
-Supramolecule #1000: Electron Cryo-microscopy of Chikungunya VLP in complex with neutr...
| Supramolecule | Name: Electron Cryo-microscopy of Chikungunya VLP in complex with neutralizing antibody Fab m242 type: sample / ID: 1000 / Number unique components: 2 |
|---|
-Supramolecule #1: Chikungunya virus
| Supramolecule | Name: Chikungunya virus / type: virus / ID: 1 / NCBI-ID: 37124 / Sci species name: Chikungunya virus / Sci species strain: 37997 / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host (natural) | Organism:  |
| Host system | Organism:  Homo sapiens (human) / Recombinant cell: HEK 293T / Recombinant plasmid: CMV/R Homo sapiens (human) / Recombinant cell: HEK 293T / Recombinant plasmid: CMV/R |
| Virus shell | Shell ID: 1 / Name: E1E2 / Diameter: 700 Å / T number (triangulation number): 4 |
-Macromolecule #1: m242 antibody Fab fragment
| Macromolecule | Name: m242 antibody Fab fragment / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Oligomeric state: monomer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human / Cell: Hybridoma Homo sapiens (human) / synonym: Human / Cell: Hybridoma |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: PBS |
| Grid | Details: 400 mesh C-flat grids |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 90 K / Instrument: GATAN CRYOPLUNGE 3 / Method: Blot for 2 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM300FEG/HE |
|---|---|
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 150,000 times magnification |
| Date | Jan 1, 2011 |
| Image recording | Digitization - Scanner: NIKON SUPER COOLSCAN 9000 / Digitization - Sampling interval: 6.35 µm / Number real images: 62 / Average electron dose: 20 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 39190 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal magnification: 38000 |
| Sample stage | Specimen holder: Side entry liquid nitrogen-cooled cryo specimen holder Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | The particles were selected using e2boxer. |
|---|---|
| CTF correction | Details: each micrograph |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 15.6 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: EMAN2 / Number images used: 1728 |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















