[English] 日本語
 Yorodumi
Yorodumi- EMDB-5566: Icosahedral reconstruction (map 1/8): Visualization of Uncorrelat... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5566 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Icosahedral reconstruction (map 1/8): Visualization of Uncorrelated Tandem Symmetry Mismatches in the Internal Genome Packaging Apparatus of a dsDNA Virus | |||||||||
 Map data Map data | Icosahedral reconstruction of bacteriophage T710A capsid I | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | bacteriophage T7 / procapsid / DNA packaging / portal / core stack / symmetry mismatch / focused asymmetric reconstruction / combinatorial assembly isomerism | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont genome ejection through host cell envelope / host cell periplasmic space / : / symbiont entry into host cell via disruption of host cell wall peptidoglycan / peptidoglycan lytic transglycosylase activity / viral portal complex / symbiont genome ejection through host cell envelope, short tail mechanism / viral DNA genome packaging / peptidoglycan metabolic process / symbiont entry into host cell via disruption of host cell envelope ...symbiont genome ejection through host cell envelope / host cell periplasmic space / : / symbiont entry into host cell via disruption of host cell wall peptidoglycan / peptidoglycan lytic transglycosylase activity / viral portal complex / symbiont genome ejection through host cell envelope, short tail mechanism / viral DNA genome packaging / peptidoglycan metabolic process / symbiont entry into host cell via disruption of host cell envelope / symbiont entry into host / viral capsid assembly / virion component / viral capsid / killing of cells of another organism / defense response to bacterium / hydrolase activity / viral translational frameshifting / host cell plasma membrane / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |   Enterobacteria phage T7 (virus) Enterobacteria phage T7 (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 7.8 Å | |||||||||
 Authors Authors | Guo F / Liu Z / Vago F / Ren Y / Wu W / Wright E / Serwer P / Jiang W | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2013 Journal: Proc Natl Acad Sci U S A / Year: 2013Title: Visualization of uncorrelated, tandem symmetry mismatches in the internal genome packaging apparatus of bacteriophage T7. Authors: Fei Guo / Zheng Liu / Frank Vago / Yue Ren / Weimin Wu / Elena T Wright / Philip Serwer / Wen Jiang /  Abstract: Motor-driven packaging of a dsDNA genome into a preformed protein capsid through a unique portal vertex is essential in the life cycle of a large number of dsDNA viruses. We have used single-particle ...Motor-driven packaging of a dsDNA genome into a preformed protein capsid through a unique portal vertex is essential in the life cycle of a large number of dsDNA viruses. We have used single-particle electron cryomicroscopy to study the multilayer structure of the portal vertex of the bacteriophage T7 procapsid, the recipient of T7 DNA in packaging. A focused asymmetric reconstruction method was developed and applied to selectively resolve neighboring pairs of symmetry-mismatched layers of the portal vertex. However, structural features in all layers of the multilayer portal vertex could not be resolved simultaneously. Our results imply that layers with mismatched symmetries can join together in several different relative orientations, and that orientations at different interfaces assort independently to produce structural isomers, a process that we call combinatorial assembly isomerism. This isomerism explains rotational smearing in previously reported asymmetric reconstructions of the portal vertex of T7 and other bacteriophages. Combinatorial assembly isomerism may represent a new regime of structural biology in which globally varying structures assemble from a common set of components. Our reconstructions collectively validate previously proposed symmetries, compositions, and sequential order of T7 portal vertex layers, resolving in tandem the 5-fold gene product 10 (gp10) shell, 12-fold gp8 portal ring, and an internal core stack consisting of 12-fold gp14 adaptor ring, 8-fold bowl-shaped gp15, and 4-fold gp16 tip. We also found a small tilt of the core stack relative to the icosahedral fivefold axis and propose that this tilt assists DNA spooling without tangling during packaging. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5566.map.gz emd_5566.map.gz | 43.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5566-v30.xml emd-5566-v30.xml emd-5566.xml emd-5566.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| Images |  T710A-CI.icos-sar-far-1to6.sections.2.png T710A-CI.icos-sar-far-1to6.sections.2.png | 877.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5566 http://ftp.pdbj.org/pub/emdb/structures/EMD-5566 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5566 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5566 | HTTPS FTP |
-Related structure data
| Related structure data |  5567C  5568C  5569C  5570C  5571C  5572C  5573C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5566.map.gz / Format: CCP4 / Size: 173.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5566.map.gz / Format: CCP4 / Size: 173.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Icosahedral reconstruction of bacteriophage T710A capsid I | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
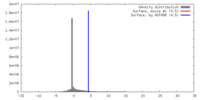
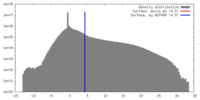
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Bacteriophage T710A capsid I
| Entire | Name: Bacteriophage T710A capsid I |
|---|---|
| Components |
|
-Supramolecule #1000: Bacteriophage T710A capsid I
| Supramolecule | Name: Bacteriophage T710A capsid I / type: sample / ID: 1000 / Number unique components: 6 |
|---|
-Macromolecule #1: gp14
| Macromolecule | Name: gp14 / type: protein_or_peptide / ID: 1 / Name.synonym: Internal virion protein B / Details: core stack protein / Number of copies: 12 / Oligomeric state: dodecamer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:   Enterobacteria phage T7 (virus) / Strain: T710A / synonym: phage T7 Enterobacteria phage T7 (virus) / Strain: T710A / synonym: phage T7 |
| Molecular weight | Theoretical: 21 KDa |
| Sequence | UniProtKB: Internal virion protein gp14 |
-Macromolecule #2: gp15
| Macromolecule | Name: gp15 / type: protein_or_peptide / ID: 2 / Name.synonym: Internal virion protein C / Details: core stack protein / Number of copies: 8 / Oligomeric state: octamer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:   Enterobacteria phage T7 (virus) / synonym: phage T7 Enterobacteria phage T7 (virus) / synonym: phage T7 |
| Molecular weight | Theoretical: 84 KDa |
| Sequence | UniProtKB: Internal virion protein gp15 |
-Macromolecule #3: gp16
| Macromolecule | Name: gp16 / type: protein_or_peptide / ID: 3 / Name.synonym: Internal virion protein D / Details: core stack protein / Number of copies: 4 / Oligomeric state: tetramer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:   Enterobacteria phage T7 (virus) / synonym: phage T7 Enterobacteria phage T7 (virus) / synonym: phage T7 |
| Molecular weight | Theoretical: 144 KDa |
| Sequence | UniProtKB: Peptidoglycan transglycosylase gp16 |
-Macromolecule #4: gp8
| Macromolecule | Name: gp8 / type: protein_or_peptide / ID: 4 / Name.synonym: Head-to-tail joining protein, portal / Details: connector/portal / Number of copies: 12 / Oligomeric state: dodecamer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:   Enterobacteria phage T7 (virus) / synonym: phage T7 Enterobacteria phage T7 (virus) / synonym: phage T7 |
| Molecular weight | Theoretical: 59 KDa |
| Sequence | UniProtKB: Portal protein |
-Macromolecule #5: gp10A
| Macromolecule | Name: gp10A / type: protein_or_peptide / ID: 5 / Name.synonym: Major capsid protein 10A Details: major capsid protein. The correct copy number is 415. Oligomeric state: icosahedral (T=7) / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:   Enterobacteria phage T7 (virus) / synonym: phage T7 Enterobacteria phage T7 (virus) / synonym: phage T7 |
| Molecular weight | Theoretical: 36 KDa |
| Sequence | UniProtKB: Major capsid protein |
-Macromolecule #6: gp9
| Macromolecule | Name: gp9 / type: protein_or_peptide / ID: 6 / Name.synonym: Capsid assembly protein / Details: scaffolding protein / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:   Enterobacteria phage T7 (virus) / synonym: phage T7 Enterobacteria phage T7 (virus) / synonym: phage T7 |
| Molecular weight | Theoretical: 34 KDa |
| Sequence | UniProtKB: Capsid assembly scaffolding protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Details: 200 mM NaCl, 10 mM Tris-HCl, 1 mM MgCl2 |
|---|---|
| Grid | Details: Ted Pella 01824, Ultrathin Carbon Film on Holey Carbon Support Film, 400 mesh, Copper |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 120 K / Instrument: FEI VITROBOT MARK I / Method: Blot for 2 seconds before plunging |
- Electron microscopy #1
Electron microscopy #1
| Microscopy ID | 1 |
|---|---|
| Microscope | FEI TITAN KRIOS |
| Temperature | Min: 80 K / Max: 105 K / Average: 100 K |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 120,000 times magnification |
| Details | Parallel beam illumination |
| Date | Sep 22, 2010 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: NIKON SUPER COOLSCAN 9000 / Digitization - Sampling interval: 6.35 µm / Number real images: 1270 / Average electron dose: 25 e/Å2 / Od range: 0.6 / Bits/pixel: 16 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 57727 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Electron microscopy #2
Electron microscopy #2
| Microscopy ID | 2 |
|---|---|
| Microscope | FEI TITAN KRIOS |
| Temperature | Min: 80 K / Max: 105 K / Average: 100 K |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 120,000 times magnification |
| Details | Parallel beam illumination |
| Date | Nov 5, 2010 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: NIKON SUPER COOLSCAN 9000 / Digitization - Sampling interval: 6.35 µm / Number real images: 1270 / Average electron dose: 25 e/Å2 / Od range: 0.6 / Bits/pixel: 16 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 57727 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | The particles were selected automatically by the ethan method and then by manual screening with the EMAN boxer program. CTF parameters were determined automatically using fitctf2.py and then visually validated using EMAN ctfit program. For 3-D reconstructions, the images were first binned 4x to make initial reconstructions. After alignment parameters and reconstructions converged, the 2x binned images were used for final reconstructions with a sampling of 2.2 A/pixel. |
|---|---|
| CTF correction | Details: phase flipping and amplitude weighted; per particle |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 7.8 Å / Resolution method: OTHER / Software - Name: jspr, EMAN2, EMAN Details: This is the Icos map of a serials of 8 maps (Icos, SAR, FAR-I to FAR-VI). Only the major capsid protein gp10 is resolved. All other proteins (gp9, gp8, gp14/15/16) are smeared. Number images used: 24980 |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)