[English] 日本語
 Yorodumi
Yorodumi- EMDB-52585: A 3.3 angstrom cryo-EM structure of an engineered high-affinity h... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | A 3.3 angstrom cryo-EM structure of an engineered high-affinity human prothrombinase complex | |||||||||
 Map data Map data | unsharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | enzyme / complex / prothrombinase / BLOOD CLOTTING | |||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to vitamin K / coagulation factor Xa / platelet alpha granule / Cargo concentration in the ER / COPII-coated ER to Golgi transport vesicle / Defective factor IX causes thrombophilia / Defective cofactor function of FVIIIa variant / Defective F9 variant does not activate FX / COPII-mediated vesicle transport / Extrinsic Pathway of Fibrin Clot Formation ...response to vitamin K / coagulation factor Xa / platelet alpha granule / Cargo concentration in the ER / COPII-coated ER to Golgi transport vesicle / Defective factor IX causes thrombophilia / Defective cofactor function of FVIIIa variant / Defective F9 variant does not activate FX / COPII-mediated vesicle transport / Extrinsic Pathway of Fibrin Clot Formation / blood circulation / positive regulation of TOR signaling / Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus / Gamma-carboxylation of protein precursors / Common Pathway of Fibrin Clot Formation / Removal of aminoterminal propeptides from gamma-carboxylated proteins / Intrinsic Pathway of Fibrin Clot Formation / endoplasmic reticulum-Golgi intermediate compartment membrane / platelet alpha granule lumen / Post-translational protein phosphorylation / phospholipid binding / Golgi lumen / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / blood coagulation / Platelet degranulation / extracellular vesicle / positive regulation of cell migration / endoplasmic reticulum lumen / copper ion binding / external side of plasma membrane / serine-type endopeptidase activity / calcium ion binding / proteolysis / extracellular space / extracellular region / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.28 Å | |||||||||
 Authors Authors | Huntington JA / Faille A / Ustok FI | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Blood / Year: 2025 Journal: Blood / Year: 2025Title: A 3.3-Å cryo-EM structure of an engineered high-affinity human prothrombinase complex. Authors: Fatma Işık Üstok / Alexandre Faille / James A Huntington /  Abstract: Thrombin is generated from prothrombin through cleavage at two sites by the enzyme prothrombinase, composed of factor (f) Xa and fVa. The affinity of fXa for fVa is low, with assembly and function ...Thrombin is generated from prothrombin through cleavage at two sites by the enzyme prothrombinase, composed of factor (f) Xa and fVa. The affinity of fXa for fVa is low, with assembly and function dependent on phospholipid (PL) membranes. Some snakes have evolved venom versions of fXa that bind to fVa with high affinity and efficiently activate prothrombin in the absence of PL. We created a similar high-affinity, PL-independent human prothrombinase with 17 mutations to human fXa (M17). The increase in affinity enabled cryogenic electron microscopy (cryo-EM) structure determination of M17-prothrombinase to a resolution of 3.3 Å. All protein domains were well resolved in the map, except for the Gla domain of fXa. The main contacts involve the serine protease and EGF2 domains of fXa and the A2 and A3 domains of fVa, resulting in the burying of a total surface area of 4,900 Å2. The map is of sufficient quality to resolve side chain interactions, including several key M17 mutations. To aid in the placement of the loop C-terminal to the A2 domain (a2-loop), we solved a high-resolution crystal structure of fXa in complex with a synthetic a2-peptide. The acidic a2-loop interacts with the basic heparin binding site of fXa, involving a conserved antiparallel b-strand interaction. The M17-prothrombinase structure is compatible with data from biochemical and mutagenesis research, and provides important new insights into the assembly and function of the prothrombinase complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_52585.map.gz emd_52585.map.gz | 162.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-52585-v30.xml emd-52585-v30.xml emd-52585.xml emd-52585.xml | 23.4 KB 23.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_52585_fsc.xml emd_52585_fsc.xml | 14.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_52585.png emd_52585.png | 74.8 KB | ||
| Filedesc metadata |  emd-52585.cif.gz emd-52585.cif.gz | 7.7 KB | ||
| Others |  emd_52585_additional_1.map.gz emd_52585_additional_1.map.gz emd_52585_half_map_1.map.gz emd_52585_half_map_1.map.gz emd_52585_half_map_2.map.gz emd_52585_half_map_2.map.gz | 304.5 MB 301.9 MB 301.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-52585 http://ftp.pdbj.org/pub/emdb/structures/EMD-52585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-52585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-52585 | HTTPS FTP |
-Related structure data
| Related structure data |  9i2hMC  9i24C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_52585.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_52585.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: sharpened map
| File | emd_52585_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_52585_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 1
| File | emd_52585_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : prothrombinase, a complex of fVa and fXa
| Entire | Name: prothrombinase, a complex of fVa and fXa |
|---|---|
| Components |
|
-Supramolecule #1: prothrombinase, a complex of fVa and fXa
| Supramolecule | Name: prothrombinase, a complex of fVa and fXa / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 Details: M17-prothrombinase was formed by mixing fVa with EGRCK-inhibited M17-fXa at a 1:6 molar ratio with a final concentration of 1.68uM in 20mM HEPES pH 7.5, 150mM NaCl and 5mM CaCl2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Activated factor Xa heavy chain
| Macromolecule | Name: Activated factor Xa heavy chain / type: protein_or_peptide / ID: 1 Details: Mutations given using chymotrypsin template numbering as in the coordinates. Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.789916 KDa |
| Recombinant expression | Organism:  Cytomegalovirus Cytomegalovirus |
| Sequence | String: IVGGQECKDG ECPWQALLIN EENEGFCGGT ILSEFYILTA AHCLYQAKRF KVRVGDRNTE QEEGGEAVHE VEVVIKHNRF TKETYDFDI AVLRLKTPIT FRMNVAPACL PERDWANETL MKQDTGIVSG FGRTHEKGRQ STRLKMLEVP YVDRHSCMLS S DFRITQNM ...String: IVGGQECKDG ECPWQALLIN EENEGFCGGT ILSEFYILTA AHCLYQAKRF KVRVGDRNTE QEEGGEAVHE VEVVIKHNRF TKETYDFDI AVLRLKTPIT FRMNVAPACL PERDWANETL MKQDTGIVSG FGRTHEKGRQ STRLKMLEVP YVDRHSCMLS S DFRITQNM FCAGYDTKQE DACQGDSGGP HVTRFKDTYF VTGIVSWGEG CARKGKYGIY TKVTRFLKWI KRSMKTRGLP KA KSHAPEV ITSSPLK UniProtKB: Coagulation factor X |
-Macromolecule #2: Factor X light chain
| Macromolecule | Name: Factor X light chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.765613 KDa |
| Recombinant expression | Organism:  Cytomegalovirus Cytomegalovirus |
| Sequence | String: ANSFLEEMKK GHLERECMEE TCSYEEAREV FEDSDKTNEF WNKYKDGDQC ETSPCQNQGK CKDGLGEYTC TCLEGFEGKN CELFTRKLC RAFNGDCDQF CKRVQSSVVC SCARGYTLAD NGKACIPTGP YPCGKQTLER UniProtKB: Coagulation factor X |
-Macromolecule #3: Coagulation factor V heavy chain
| Macromolecule | Name: Coagulation factor V heavy chain / type: protein_or_peptide / ID: 3 / Details: TYS is a sulphated Tyrosine. / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 81.354453 KDa |
| Recombinant expression | Organism: unidentified plasmid (others) |
| Sequence | String: AQLRQFYVAA QGISWSYRPE PTNSSLNLSV TSFKKIVYRE YEPYFKKEKP QSTISGLLGP TLYAEVGDII KVHFKNKADK PLSIHPQGI RYSKLSEGAS YLDHTFPAEK MDDAVAPGRE YTYEWSISED SGPTHDDPPC LTHIYYSHEN LIEDFNSGLI G PLLICKKG ...String: AQLRQFYVAA QGISWSYRPE PTNSSLNLSV TSFKKIVYRE YEPYFKKEKP QSTISGLLGP TLYAEVGDII KVHFKNKADK PLSIHPQGI RYSKLSEGAS YLDHTFPAEK MDDAVAPGRE YTYEWSISED SGPTHDDPPC LTHIYYSHEN LIEDFNSGLI G PLLICKKG TLTEGGTQKT FDKQIVLLFA VFDESKSWSQ SSSLMYTVNG YVNGTMPDIT VCAHDHISWH LLGMSSGPEL FS IHFNGQV LEQNHHKVSA ITLVSATSTT ANMTVGPEGK WIISSLTPKH LQAGMQAYID IKNCPKKTRN LKKITREQRR HMK RWEYFI AAEEVIWDYA PVIPANMDKK YRSQHLDNFS NQIGKHYKKV MYTQYEDESF TKHTVNPNMK EDGILGPIIR AQVR DTLKI VFKNMASRPY SIYPHGVTFS PYEDEVNSSF TSGRNNTMIR AVQPGETYTY KWNILEFDEP TENDAQCLTR PYYSD VDIM RDIASGLIGL LLICKSRSLD RRGIQRAADI EQQAVFAVFD ENKSWYLEDN INKFCENPDE VKRDDPKFYE SNIMST ING YVPESITTLG FCFDDTVQWH FCSVGTQNEI LTIHFTGHSF IYGKRHEDTL TLFPMRGESV TVTMDNVGTW MLTSMNS SP RSKKLRLKFR DVKCIPDDDE DS(TYS)EIFEPPE STVMATRKMH DRLEPEDEES DADYDYQNRL AAALGIR UniProtKB: Coagulation factor V |
-Macromolecule #4: Coagulation factor V light chain
| Macromolecule | Name: Coagulation factor V light chain / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 75.283008 KDa |
| Recombinant expression | Organism: unidentified plasmid (others) |
| Sequence | String: SNNGNRRNYY IAAEEISWDY SEFVQRETDI EDSDDIPEDT TYKKVVFRKY LDSTFTKRDP RGEYEEHLGI LGPIIRAEVD DVIQVRFKN LASRPYSLHA HGLSYEKSSE GKTYEDDSPE WFKEDNAVQP NSSYTYVWHA TERSGPESPG SACRAWAYYS A VNPEKDIH ...String: SNNGNRRNYY IAAEEISWDY SEFVQRETDI EDSDDIPEDT TYKKVVFRKY LDSTFTKRDP RGEYEEHLGI LGPIIRAEVD DVIQVRFKN LASRPYSLHA HGLSYEKSSE GKTYEDDSPE WFKEDNAVQP NSSYTYVWHA TERSGPESPG SACRAWAYYS A VNPEKDIH SGLIGPLLIC QKGILHKDSN MPMDMREFVL LFMTFDEKKS WYYEKKSRSS WRLTSSEMKK SHEFHAINGM IY SLPGLKM YEQEWVRLHL LNIGGSQDIH VVHFHGQTLL ENGNKQHQLG VWPLLPGSFK TLEMKASKPG WWLLNTEVGE NQR AGMQTP FLIMDRDCRM PMGLSTGIIS DSQIKASEFL GYWEPRLARL NNGGSYNAWS VEKLAAEFAS KPWIQVDMQK EVII TGIQT QGAKHYLKSC YTTEFYVAYS SNQINWQIFK GNSTRNVMYF NGNSDASTIK ENQFDPPIVA RYIRISPTRA YNRPT LRLE LQGCEVNGCS TPLGMENGKI ENKQITASSF KKSWWGDYWE PFRARLNAQG RVNAWQAKAN NNKQWLEIDL LKIKKI TAI ITQGCKSLSS EMYVKSYTIH YSEQGVEWKP YRLKSSMVDK IFEGNTNTKG HVKNFFNPPI ISRFIRVIPK TWNQSIA LR LELFGCDIY UniProtKB: Coagulation factor V |
-Macromolecule #9: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 9 / Number of copies: 2 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #10: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 10 / Number of copies: 1 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #11: L-alpha-glutamyl-N-{(1S)-4-{[amino(iminio)methyl]amino}-1-[(1S)-2...
| Macromolecule | Name: L-alpha-glutamyl-N-{(1S)-4-{[amino(iminio)methyl]amino}-1-[(1S)-2-chloro-1-hydroxyethyl]butyl}glycinamide type: ligand / ID: 11 / Number of copies: 1 / Formula: 0GJ |
|---|---|
| Molecular weight | Theoretical: 395.862 Da |
| Chemical component information | 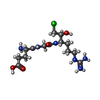 ChemComp-0GJ: |
-Macromolecule #12: COPPER (II) ION
| Macromolecule | Name: COPPER (II) ION / type: ligand / ID: 12 / Number of copies: 1 / Formula: CU |
|---|---|
| Molecular weight | Theoretical: 63.546 Da |
| Chemical component information |  ChemComp-CU: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Details: 20mM HEPES, 150mM NaCl and 5mM CaCl2 |
|---|---|
| Grid | Model: Quantifoil R0.6/1 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.025 kPa / Details: using a Pelco EasiGlow |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
| Details | M17-prothrombinase was formed by mixing fVa with EGRCK-inhibited M17-fXa at a 1:6 molar ratio with a final concentration of 1.68uM |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 45.74 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)













































