+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5197 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of human mTORC1 | |||||||||
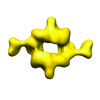
 Map data Map data | Density map of human mTORC1 resolution filtered to 26 Angstroms | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Kinase complex / metabolic role | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 26.0 Å | |||||||||
 Authors Authors | Yip CK / Murata K / Walz T / Sabataini DM / Kang SA | |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2010 Journal: Mol Cell / Year: 2010Title: Structure of the human mTOR complex I and its implications for rapamycin inhibition. Authors: Calvin K Yip / Kazuyoshi Murata / Thomas Walz / David M Sabatini / Seong A Kang /  Abstract: The mammalian target of rapamycin complex 1 (mTORC1) regulates cell growth in response to the nutrient and energy status of the cell, and its deregulation is common in human cancers. Little is known ...The mammalian target of rapamycin complex 1 (mTORC1) regulates cell growth in response to the nutrient and energy status of the cell, and its deregulation is common in human cancers. Little is known about the overall architecture and subunit organization of this essential signaling complex. We have determined the three-dimensional (3D) structure of the fully assembled human mTORC1 by cryo-electron microscopy (cryo-EM). Our analyses reveal that mTORC1 is an obligate dimer with an overall rhomboid shape and a central cavity. The dimeric interfaces are formed by interlocking interactions between the mTOR and raptor subunits. Extended incubation with FKBP12-rapamycin compromises the structural integrity of mTORC1 in a stepwise manner, leading us to propose a model in which rapamycin inhibits mTORC1-mediated phosphorylation of 4E-BP1 and S6K1 through different mechanisms. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5197.map.gz emd_5197.map.gz | 4.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5197-v30.xml emd-5197-v30.xml emd-5197.xml emd-5197.xml | 13.1 KB 13.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5197_1.tif emd_5197_1.tif | 1.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5197 http://ftp.pdbj.org/pub/emdb/structures/EMD-5197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5197 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5197.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5197.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Density map of human mTORC1 resolution filtered to 26 Angstroms | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Purified human mTORC1
| Entire | Name: Purified human mTORC1 |
|---|---|
| Components |
|
-Supramolecule #1000: Purified human mTORC1
| Supramolecule | Name: Purified human mTORC1 / type: sample / ID: 1000 / Oligomeric state: dimer of heterotetramer / Number unique components: 4 |
|---|---|
| Molecular weight | Theoretical: 1.0 MDa |
-Macromolecule #1: mTOR
| Macromolecule | Name: mTOR / type: protein_or_peptide / ID: 1 / Name.synonym: mTOR / Number of copies: 2 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: human / Cell: HEK-293T / Location in cell: cytoplasm Homo sapiens (human) / synonym: human / Cell: HEK-293T / Location in cell: cytoplasm |
| Molecular weight | Theoretical: 289 KDa |
| Recombinant expression | Organism: Mammalian cells |
-Macromolecule #2: raptor
| Macromolecule | Name: raptor / type: protein_or_peptide / ID: 2 / Name.synonym: raptor / Number of copies: 2 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: human / Cell: HEK-293T / Location in cell: cytoplasm Homo sapiens (human) / synonym: human / Cell: HEK-293T / Location in cell: cytoplasm |
| Molecular weight | Theoretical: 150 KDa |
| Recombinant expression | Organism: Mammalian cells |
-Macromolecule #3: mLST8
| Macromolecule | Name: mLST8 / type: protein_or_peptide / ID: 3 / Name.synonym: mLST8 / Number of copies: 2 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human / Cell: HEK-293T / Location in cell: cytoplasm Homo sapiens (human) / synonym: Human / Cell: HEK-293T / Location in cell: cytoplasm |
| Molecular weight | Experimental: 36 KDa / Theoretical: 36 KDa |
| Recombinant expression | Organism: Mammalian cells |
-Macromolecule #4: PRAS40
| Macromolecule | Name: PRAS40 / type: protein_or_peptide / ID: 4 / Name.synonym: PRAS40 / Number of copies: 2 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human / Cell: HEK-293T / Location in cell: cytoplasm Homo sapiens (human) / synonym: Human / Cell: HEK-293T / Location in cell: cytoplasm |
| Molecular weight | Experimental: 27 KDa / Theoretical: 27 KDa |
| Recombinant expression | Organism: Mammalian cells |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.02 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 50mM HEPES, 150mM NaCl |
| Grid | Details: 400 mesh Quantifoil R1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Instrument: OTHER / Details: Vitrification instrument: Vitrobot / Method: Blot for 2 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Date | Mar 12, 2008 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 21 µm / Number real images: 323 |
| Tilt angle min | 0 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 51159 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 1.4 mm / Nominal defocus max: 6.0 µm / Nominal defocus min: 2.5 µm / Nominal magnification: 52000 |
| Sample stage | Specimen holder: Side entry liquid nitrogen-cooled cryo specimen holder Specimen holder model: GATAN LIQUID NITROGEN / Tilt angle max: 45 |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Details | PDBEntryID_givenInChain. Protocol: manual docking. model was manually docked into density map using Chimera |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: rigid body |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















