+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-5186 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of an apoptosome-procaspase-9 CARD complex | |||||||||
 マップデータ マップデータ | Structure of the human apoptosome with procaspase-9 CARD | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | apoptosome / Apaf-1 / procaspase-9 CARD / apoptosis | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報: / Release of apoptotic factors from the mitochondria / Formation of apoptosome / Activation of caspases through apoptosome-mediated cleavage / Pyroptosis / Regulation of the apoptosome activity / Transcriptional activation of mitochondrial biogenesis / response to G1 DNA damage checkpoint signaling / regulation of apoptotic DNA fragmentation / Formation of apoptosome ...: / Release of apoptotic factors from the mitochondria / Formation of apoptosome / Activation of caspases through apoptosome-mediated cleavage / Pyroptosis / Regulation of the apoptosome activity / Transcriptional activation of mitochondrial biogenesis / response to G1 DNA damage checkpoint signaling / regulation of apoptotic DNA fragmentation / Formation of apoptosome / Detoxification of Reactive Oxygen Species / apoptosome / TP53 Regulates Metabolic Genes / Cytoprotection by HMOX1 / cysteine-type endopeptidase activator activity / Respiratory electron transport / Activation of caspases through apoptosome-mediated cleavage / Regulation of the apoptosome activity / SMAC (DIABLO) binds to IAPs / SMAC(DIABLO)-mediated dissociation of IAP:caspase complexes / mitochondrial electron transport, cytochrome c to oxygen / cysteine-type endopeptidase activator activity involved in apoptotic process / mitochondrial electron transport, ubiquinol to cytochrome c / TP53 Regulates Transcription of Caspase Activators and Caspases / Transcriptional Regulation by E2F6 / forebrain development / intrinsic apoptotic signaling pathway in response to endoplasmic reticulum stress / cellular response to transforming growth factor beta stimulus / heat shock protein binding / response to nutrient / cardiac muscle cell apoptotic process / intrinsic apoptotic signaling pathway / positive regulation of apoptotic signaling pathway / neural tube closure / kidney development / ADP binding / mitochondrial intermembrane space / nervous system development / neuron apoptotic process / secretory granule lumen / regulation of apoptotic process / ficolin-1-rich granule lumen / cell differentiation / response to hypoxia / electron transfer activity / positive regulation of apoptotic process / nucleotide binding / apoptotic process / heme binding / Neutrophil degranulation / protein-containing complex / extracellular exosome / extracellular region / ATP binding / metal ion binding / identical protein binding / nucleus / cytosol 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /  | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 9.5 Å | |||||||||
 データ登録者 データ登録者 | Yuan S / Yu X / Topf M / Ludtke SJ / Wang X / Akey CW | |||||||||
 引用 引用 |  ジャーナル: Structure / 年: 2010 ジャーナル: Structure / 年: 2010タイトル: Structure of an apoptosome-procaspase-9 CARD complex. 著者: Shujun Yuan / Xinchao Yu / Maya Topf / Steven J Ludtke / Xiaodong Wang / Christopher W Akey /  要旨: Apaf-1 coassembles with cytochrome c to form the apoptosome, which then binds and activates procaspase-9 (pc-9). We removed pc-9 catalytic domains from the holoapoptosome by site-directed ...Apaf-1 coassembles with cytochrome c to form the apoptosome, which then binds and activates procaspase-9 (pc-9). We removed pc-9 catalytic domains from the holoapoptosome by site-directed thrombinolysis. A structure of the resulting apoptosome-pc-9 CARD complex was then determined at approximately 9.5 A resolution. In our model, the central hub is constructed like other AAA+ protein rings but also contains novel features. At higher radius, the regulatory region of each Apaf-1 is comprised of tandem seven and eight blade beta-propellers with cytochrome c docked between them. Remarkably, Apaf-1 CARDs are disordered in the ground state. During activation, each Apaf-1 CARD interacts with a pc-9 CARD and these heterodimers form a flexibly tethered "disk" that sits above the central hub. When taken together, the data reveal conformational changes during Apaf-1 assembly that allow pc-9 activation. The model also provides a plausible explanation for the effects of NOD mutations that have been mapped onto the central hub. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_5186.map.gz emd_5186.map.gz | 2.4 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-5186-v30.xml emd-5186-v30.xml emd-5186.xml emd-5186.xml | 16.4 KB 16.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_5186_1.jpg emd_5186_1.jpg | 202.5 KB | ||
| マスクデータ |  emd_5186_msk_1.map emd_5186_msk_1.map | 30.5 MB |  マスクマップ マスクマップ | |
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5186 http://ftp.pdbj.org/pub/emdb/structures/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5186 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_5186_validation.pdf.gz emd_5186_validation.pdf.gz | 334.2 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_5186_full_validation.pdf.gz emd_5186_full_validation.pdf.gz | 333.8 KB | 表示 | |
| XML形式データ |  emd_5186_validation.xml.gz emd_5186_validation.xml.gz | 5.7 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_5186.map.gz / 形式: CCP4 / 大きさ: 29.8 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_5186.map.gz / 形式: CCP4 / 大きさ: 29.8 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Structure of the human apoptosome with procaspase-9 CARD | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 2.26 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
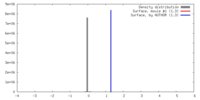
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-セグメンテーションマップ: This is a mask used to filter the final 3D volume
| 注釈 | This is a mask used to filter the final 3D volume | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ファイル |  emd_5186_msk_1.map emd_5186_msk_1.map | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
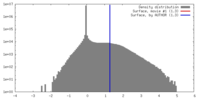
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : human apoptosome with bound procaspase-9 CARD
| 全体 | 名称: human apoptosome with bound procaspase-9 CARD |
|---|---|
| 要素 |
|
-超分子 #1000: human apoptosome with bound procaspase-9 CARD
| 超分子 | 名称: human apoptosome with bound procaspase-9 CARD / タイプ: sample / ID: 1000 詳細: Apoptosomes were assembled in low salt buffer, procaspase-9 with a thrombin site in the CARD-p20 linker was added, and then the complex was thrombinized to release pc-9 catalytic domains. 集合状態: heptameric Apaf-1 in the apoptosome with 7 bound procaspase-9 CARDs Number unique components: 3 |
|---|---|
| 分子量 | 理論値: 1.1 MDa |
-分子 #1: Apaf-1
| 分子 | 名称: Apaf-1 / タイプ: protein_or_peptide / ID: 1 / Name.synonym: Apaf-1 詳細: Seven Apaf-1 molecules were assembled with cytochrome c and dATP to form an apoptosome complex. コピー数: 7 / 集合状態: heptamer / 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) / 別称: Human / 細胞中の位置: cytosol Homo sapiens (ヒト) / 別称: Human / 細胞中の位置: cytosol |
| 分子量 | 理論値: 135 KDa |
| 組換発現 | 生物種: sf21 insect cells (unknown) / 組換プラスミド: pFastBac1 |
| 配列 | GO: GO: 0008635 / InterPro: Apoptotic protease-activating factor 1 |
-分子 #2: procaspase-9
| 分子 | 名称: procaspase-9 / タイプ: protein_or_peptide / ID: 2 / Name.synonym: procaspase-9 詳細: procaspase-9 binds on the human apoptosome through CARD-CARD interactions. Procaspase-9 was added to the apoptosome in slight excess and then thrombinized to remove the catalytic domains. 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) / 別称: Human / 細胞中の位置: cytosol Homo sapiens (ヒト) / 別称: Human / 細胞中の位置: cytosol |
| 分子量 | 理論値: 10 KDa |
| 組換発現 | 生物種:  |
-分子 #3: cytochrome c
| 分子 | 名称: cytochrome c / タイプ: protein_or_peptide / ID: 3 / Name.synonym: cytochrome c / 詳細: cytochrome c is the assembly activator for Apaf-1 / コピー数: 7 / 集合状態: monomer / 組換発現: No / データベース: NCBI |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 10 KDa |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 3 mg/mL |
|---|---|
| 緩衝液 | pH: 7.5 詳細: 20mM HEPES, 10mM KCl, 1.5mM MgCl2, 1mM EDTA, 1mM EGTA, 1mM DTT |
| グリッド | 詳細: Quantifoil R1.2/1.3 holey grids |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 77 K / 装置: FEI VITROBOT MARK III / 詳細: Vitrification instrument: Vitrobot Mark 3 (FEI) / 手法: Blot for 2-3 seconds before plunging at 20C |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI F20 |
|---|---|
| 温度 | 平均: 96 K |
| アライメント法 | Legacy - 非点収差: objective lens astigmatism was corrected at 200,000 times magnification |
| 日付 | 2008年8月1日 |
| 撮影 | カテゴリ: FILM / フィルム・検出器のモデル: KODAK SO-163 FILM / デジタル化 - スキャナー: ZEISS SCAI / デジタル化 - サンプリング間隔: 7 µm / 実像数: 400 / 平均電子線量: 25 e/Å2 / Od range: 1 |
| 電子線 | 加速電圧: 120 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 62000 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2 mm / 最大 デフォーカス(公称値): 3.0 µm / 最小 デフォーカス(公称値): 1.5 µm / 倍率(公称値): 62000 |
| 試料ステージ | 試料ホルダー: Side entry liquid nitrogen-cooled cryo specimen holder. 試料ホルダーモデル: GATAN LIQUID NITROGEN |
| 実験機器 |  モデル: Tecnai F20 / 画像提供: FEI Company |
- 画像解析
画像解析
| 詳細 | particles were selected with boxer |
|---|---|
| CTF補正 | 詳細: Each image |
| 最終 再構成 | アルゴリズム: OTHER / 解像度のタイプ: BY AUTHOR / 解像度: 9.5 Å / 解像度の算出法: OTHER / ソフトウェア - 名称: EMAN 詳細: Final map contains 34000 particles. Setsf filtration was applied to the final map to boost amplitude at high resolution range and in the last cycles of refinement. 使用した粒子像数: 42000 |
| 最終 2次元分類 | クラス数: 592 |
-原子モデル構築 1
| 初期モデル | PDB ID: Chain - Chain ID: A |
|---|---|
| ソフトウェア | 名称:  chimera chimera |
| 詳細 | PDBEntryID_givenInChain. Protocol: rigid body for each domain. After chimera fitting, Flex-EM was used to improve fitting and minimize collisions. The beta propellers were modeled using the map as a restraint, sequence alignments and the crystal structure of actin interacting protein 1. |
| 精密化 | 空間: REAL / プロトコル: RIGID BODY FIT / 当てはまり具合の基準: cross correlation |
| 得られたモデル |  PDB-3j2t: |
 ムービー
ムービー コントローラー
コントローラー



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)