+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5186 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of an apoptosome-procaspase-9 CARD complex | |||||||||
 Map data Map data | Structure of the human apoptosome with procaspase-9 CARD | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | apoptosome / Apaf-1 / procaspase-9 CARD / apoptosis | |||||||||
| Function / homology |  Function and homology information Function and homology information: / Release of apoptotic factors from the mitochondria / Formation of apoptosome / Activation of caspases through apoptosome-mediated cleavage / Pyroptosis / Regulation of the apoptosome activity / Transcriptional activation of mitochondrial biogenesis / response to G1 DNA damage checkpoint signaling / regulation of apoptotic DNA fragmentation / Formation of apoptosome ...: / Release of apoptotic factors from the mitochondria / Formation of apoptosome / Activation of caspases through apoptosome-mediated cleavage / Pyroptosis / Regulation of the apoptosome activity / Transcriptional activation of mitochondrial biogenesis / response to G1 DNA damage checkpoint signaling / regulation of apoptotic DNA fragmentation / Formation of apoptosome / Detoxification of Reactive Oxygen Species / apoptosome / TP53 Regulates Metabolic Genes / Cytoprotection by HMOX1 / cysteine-type endopeptidase activator activity / Respiratory electron transport / Activation of caspases through apoptosome-mediated cleavage / Regulation of the apoptosome activity / SMAC (DIABLO) binds to IAPs / SMAC(DIABLO)-mediated dissociation of IAP:caspase complexes / mitochondrial electron transport, cytochrome c to oxygen / cysteine-type endopeptidase activator activity involved in apoptotic process / mitochondrial electron transport, ubiquinol to cytochrome c / TP53 Regulates Transcription of Caspase Activators and Caspases / forebrain development / Transcriptional Regulation by E2F6 / intrinsic apoptotic signaling pathway in response to endoplasmic reticulum stress / cellular response to transforming growth factor beta stimulus / heat shock protein binding / cardiac muscle cell apoptotic process / response to nutrient / intrinsic apoptotic signaling pathway / positive regulation of apoptotic signaling pathway / neural tube closure / kidney development / ADP binding / mitochondrial intermembrane space / nervous system development / neuron apoptotic process / secretory granule lumen / regulation of apoptotic process / ficolin-1-rich granule lumen / response to hypoxia / cell differentiation / electron transfer activity / positive regulation of apoptotic process / nucleotide binding / heme binding / apoptotic process / Neutrophil degranulation / protein-containing complex / extracellular exosome / extracellular region / ATP binding / metal ion binding / identical protein binding / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.5 Å | |||||||||
 Authors Authors | Yuan S / Yu X / Topf M / Ludtke SJ / Wang X / Akey CW | |||||||||
 Citation Citation |  Journal: Structure / Year: 2010 Journal: Structure / Year: 2010Title: Structure of an apoptosome-procaspase-9 CARD complex. Authors: Shujun Yuan / Xinchao Yu / Maya Topf / Steven J Ludtke / Xiaodong Wang / Christopher W Akey /  Abstract: Apaf-1 coassembles with cytochrome c to form the apoptosome, which then binds and activates procaspase-9 (pc-9). We removed pc-9 catalytic domains from the holoapoptosome by site-directed ...Apaf-1 coassembles with cytochrome c to form the apoptosome, which then binds and activates procaspase-9 (pc-9). We removed pc-9 catalytic domains from the holoapoptosome by site-directed thrombinolysis. A structure of the resulting apoptosome-pc-9 CARD complex was then determined at approximately 9.5 A resolution. In our model, the central hub is constructed like other AAA+ protein rings but also contains novel features. At higher radius, the regulatory region of each Apaf-1 is comprised of tandem seven and eight blade beta-propellers with cytochrome c docked between them. Remarkably, Apaf-1 CARDs are disordered in the ground state. During activation, each Apaf-1 CARD interacts with a pc-9 CARD and these heterodimers form a flexibly tethered "disk" that sits above the central hub. When taken together, the data reveal conformational changes during Apaf-1 assembly that allow pc-9 activation. The model also provides a plausible explanation for the effects of NOD mutations that have been mapped onto the central hub. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5186.map.gz emd_5186.map.gz | 2.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5186-v30.xml emd-5186-v30.xml emd-5186.xml emd-5186.xml | 16.4 KB 16.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5186_1.jpg emd_5186_1.jpg | 202.5 KB | ||
| Masks |  emd_5186_msk_1.map emd_5186_msk_1.map | 30.5 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5186 http://ftp.pdbj.org/pub/emdb/structures/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5186 | HTTPS FTP |
-Validation report
| Summary document |  emd_5186_validation.pdf.gz emd_5186_validation.pdf.gz | 334.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5186_full_validation.pdf.gz emd_5186_full_validation.pdf.gz | 333.8 KB | Display | |
| Data in XML |  emd_5186_validation.xml.gz emd_5186_validation.xml.gz | 5.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5186 | HTTPS FTP |
-Related structure data
| Related structure data |  3j2tM M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_5186.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5186.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of the human apoptosome with procaspase-9 CARD | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.26 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Segmentation: This is a mask used to filter the final 3D volume
| Annotation | This is a mask used to filter the final 3D volume | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_5186_msk_1.map emd_5186_msk_1.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
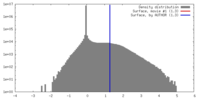
| Density Histograms |
- Sample components
Sample components
-Entire : human apoptosome with bound procaspase-9 CARD
| Entire | Name: human apoptosome with bound procaspase-9 CARD |
|---|---|
| Components |
|
-Supramolecule #1000: human apoptosome with bound procaspase-9 CARD
| Supramolecule | Name: human apoptosome with bound procaspase-9 CARD / type: sample / ID: 1000 Details: Apoptosomes were assembled in low salt buffer, procaspase-9 with a thrombin site in the CARD-p20 linker was added, and then the complex was thrombinized to release pc-9 catalytic domains. Oligomeric state: heptameric Apaf-1 in the apoptosome with 7 bound procaspase-9 CARDs Number unique components: 3 |
|---|---|
| Molecular weight | Theoretical: 1.1 MDa |
-Macromolecule #1: Apaf-1
| Macromolecule | Name: Apaf-1 / type: protein_or_peptide / ID: 1 / Name.synonym: Apaf-1 Details: Seven Apaf-1 molecules were assembled with cytochrome c and dATP to form an apoptosome complex. Number of copies: 7 / Oligomeric state: heptamer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human / Location in cell: cytosol Homo sapiens (human) / synonym: Human / Location in cell: cytosol |
| Molecular weight | Theoretical: 135 KDa |
| Recombinant expression | Organism: sf21 insect cells (unknown) / Recombinant plasmid: pFastBac1 |
| Sequence | GO: GO: 0008635 / InterPro: Apoptotic protease-activating factor 1 |
-Macromolecule #2: procaspase-9
| Macromolecule | Name: procaspase-9 / type: protein_or_peptide / ID: 2 / Name.synonym: procaspase-9 Details: procaspase-9 binds on the human apoptosome through CARD-CARD interactions. Procaspase-9 was added to the apoptosome in slight excess and then thrombinized to remove the catalytic domains. Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human / Location in cell: cytosol Homo sapiens (human) / synonym: Human / Location in cell: cytosol |
| Molecular weight | Theoretical: 10 KDa |
| Recombinant expression | Organism:  |
-Macromolecule #3: cytochrome c
| Macromolecule | Name: cytochrome c / type: protein_or_peptide / ID: 3 / Name.synonym: cytochrome c / Details: cytochrome c is the assembly activator for Apaf-1 / Number of copies: 7 / Oligomeric state: monomer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 10 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 20mM HEPES, 10mM KCl, 1.5mM MgCl2, 1mM EDTA, 1mM EGTA, 1mM DTT |
| Grid | Details: Quantifoil R1.2/1.3 holey grids |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 77 K / Instrument: FEI VITROBOT MARK III / Details: Vitrification instrument: Vitrobot Mark 3 (FEI) / Method: Blot for 2-3 seconds before plunging at 20C |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Average: 96 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected at 200,000 times magnification |
| Date | Aug 1, 2008 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 400 / Average electron dose: 25 e/Å2 / Od range: 1 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 62000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 62000 |
| Sample stage | Specimen holder: Side entry liquid nitrogen-cooled cryo specimen holder. Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | particles were selected with boxer |
|---|---|
| CTF correction | Details: Each image |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 9.5 Å / Resolution method: OTHER / Software - Name: EMAN Details: Final map contains 34000 particles. Setsf filtration was applied to the final map to boost amplitude at high resolution range and in the last cycles of refinement. Number images used: 42000 |
| Final two d classification | Number classes: 592 |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Software | Name:  chimera chimera |
| Details | PDBEntryID_givenInChain. Protocol: rigid body for each domain. After chimera fitting, Flex-EM was used to improve fitting and minimize collisions. The beta propellers were modeled using the map as a restraint, sequence alignments and the crystal structure of actin interacting protein 1. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: cross correlation |
| Output model |  PDB-3j2t: |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)