[English] 日本語
 Yorodumi
Yorodumi- EMDB-51429: Structure of HECT E3 TRIP12 forming K29/K48-branched Ubiquitin chains -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of HECT E3 TRIP12 forming K29/K48-branched Ubiquitin chains | |||||||||
 Map data Map data | deepEMhancer-sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | LIGASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationheterochromatin boundary formation / HECT-type E3 ubiquitin transferase / hypothalamus gonadotrophin-releasing hormone neuron development / nuclear thyroid hormone receptor binding / female meiosis I / positive regulation of protein monoubiquitination / fat pad development / mitochondrion transport along microtubule / female gonad development / DNA repair-dependent chromatin remodeling ...heterochromatin boundary formation / HECT-type E3 ubiquitin transferase / hypothalamus gonadotrophin-releasing hormone neuron development / nuclear thyroid hormone receptor binding / female meiosis I / positive regulation of protein monoubiquitination / fat pad development / mitochondrion transport along microtubule / female gonad development / DNA repair-dependent chromatin remodeling / seminiferous tubule development / male meiosis I / regulation of embryonic development / positive regulation of intrinsic apoptotic signaling pathway by p53 class mediator / energy homeostasis / neuron projection morphogenesis / regulation of proteasomal protein catabolic process / Maturation of protein E / Maturation of protein E / ER Quality Control Compartment (ERQC) / Myoclonic epilepsy of Lafora / FLT3 signaling by CBL mutants / Constitutive Signaling by NOTCH1 HD Domain Mutants / IRAK2 mediated activation of TAK1 complex / Prevention of phagosomal-lysosomal fusion / Alpha-protein kinase 1 signaling pathway / Glycogen synthesis / IRAK1 recruits IKK complex / IRAK1 recruits IKK complex upon TLR7/8 or 9 stimulation / Endosomal Sorting Complex Required For Transport (ESCRT) / Membrane binding and targetting of GAG proteins / Negative regulation of FLT3 / Regulation of TBK1, IKKε (IKBKE)-mediated activation of IRF3, IRF7 / PTK6 Regulates RTKs and Their Effectors AKT1 and DOK1 / Regulation of TBK1, IKKε-mediated activation of IRF3, IRF7 upon TLR3 ligation / IRAK2 mediated activation of TAK1 complex upon TLR7/8 or 9 stimulation / NOTCH2 Activation and Transmission of Signal to the Nucleus / TICAM1,TRAF6-dependent induction of TAK1 complex / TICAM1-dependent activation of IRF3/IRF7 / APC/C:Cdc20 mediated degradation of Cyclin B / Regulation of FZD by ubiquitination / Downregulation of ERBB4 signaling / APC-Cdc20 mediated degradation of Nek2A / p75NTR recruits signalling complexes / InlA-mediated entry of Listeria monocytogenes into host cells / TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling / Regulation of pyruvate metabolism / NF-kB is activated and signals survival / TRAF6-mediated induction of TAK1 complex within TLR4 complex / regulation of neuron apoptotic process / Downregulation of ERBB2:ERBB3 signaling / Regulation of innate immune responses to cytosolic DNA / Pexophagy / NRIF signals cell death from the nucleus / Activated NOTCH1 Transmits Signal to the Nucleus / Regulation of PTEN localization / VLDLR internalisation and degradation / Synthesis of active ubiquitin: roles of E1 and E2 enzymes / positive regulation of protein ubiquitination / TICAM1, RIP1-mediated IKK complex recruitment / Regulation of BACH1 activity / Translesion synthesis by REV1 / MAP3K8 (TPL2)-dependent MAPK1/3 activation / Degradation of CDH1 / Translesion synthesis by POLK / InlB-mediated entry of Listeria monocytogenes into host cell / JNK (c-Jun kinases) phosphorylation and activation mediated by activated human TAK1 / Activation of IRF3, IRF7 mediated by TBK1, IKKε (IKBKE) / Josephin domain DUBs / Downregulation of TGF-beta receptor signaling / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / IKK complex recruitment mediated by RIP1 / Degradation of CRY and PER proteins / Regulation of activated PAK-2p34 by proteasome mediated degradation / PINK1-PRKN Mediated Mitophagy / regulation of mitochondrial membrane potential / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / TNFR1-induced NF-kappa-B signaling pathway / Autodegradation of Cdh1 by Cdh1:APC/C / TCF dependent signaling in response to WNT / Regulation of NF-kappa B signaling / APC/C:Cdc20 mediated degradation of Securin / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / activated TAK1 mediates p38 MAPK activation / Asymmetric localization of PCP proteins / Ubiquitin-dependent degradation of Cyclin D / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / Regulation of signaling by CBL / TNFR2 non-canonical NF-kB pathway / AUF1 (hnRNP D0) binds and destabilizes mRNA / NOTCH3 Activation and Transmission of Signal to the Nucleus / Negative regulators of DDX58/IFIH1 signaling / Assembly of the pre-replicative complex / Negative regulation of FGFR3 signaling / Fanconi Anemia Pathway / Peroxisomal protein import / Vpu mediated degradation of CD4 / Deactivation of the beta-catenin transactivating complex Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.69 Å | |||||||||
 Authors Authors | Maiwald SA / Schulman BA | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2025 Journal: Nat Struct Mol Biol / Year: 2025Title: TRIP12 structures reveal HECT E3 formation of K29 linkages and branched ubiquitin chains. Authors: Samuel A Maiwald / Laura A Schneider / Ronnald Vollrath / Joanna Liwocha / Matthew D Maletic / Kirby N Swatek / Monique P C Mulder / Brenda A Schulman /      Abstract: Regulation by ubiquitin depends on E3 ligases forging chains of specific topologies, yet the mechanisms underlying the generation of atypical linkages remain largely elusive. Here we utilize ...Regulation by ubiquitin depends on E3 ligases forging chains of specific topologies, yet the mechanisms underlying the generation of atypical linkages remain largely elusive. Here we utilize biochemistry, chemistry, and cryo-EM to define the catalytic architecture producing K29 linkages and K29/K48 branches for the human HECT E3 TRIP12. TRIP12 resembles a pincer. One pincer side comprises tandem ubiquitin-binding domains, engaging the proximal ubiquitin to direct its K29 towards the ubiquitylation active site, and selectively capturing a distal ubiquitin from a K48-linked chain. The opposite pincer side-the HECT domain-precisely juxtaposes the ubiquitins to be joined, further ensuring K29 linkage specificity. Comparison to the prior structure visualizing K48-linked chain formation by UBR5 reveals a similar mechanism shared by two human HECT enzymes: parallel features of the E3s, donor and acceptor ubiquitins configure the active site around the targeted lysine, with E3-specific domains buttressing the acceptor for linkage-specific polyubiquitylation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_51429.map.gz emd_51429.map.gz | 106.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-51429-v30.xml emd-51429-v30.xml emd-51429.xml emd-51429.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_51429_fsc.xml emd_51429_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_51429.png emd_51429.png | 124.5 KB | ||
| Masks |  emd_51429_msk_1.map emd_51429_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-51429.cif.gz emd-51429.cif.gz | 7.1 KB | ||
| Others |  emd_51429_additional_1.map.gz emd_51429_additional_1.map.gz emd_51429_half_map_1.map.gz emd_51429_half_map_1.map.gz emd_51429_half_map_2.map.gz emd_51429_half_map_2.map.gz | 61.9 MB 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-51429 http://ftp.pdbj.org/pub/emdb/structures/EMD-51429 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51429 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51429 | HTTPS FTP |
-Related structure data
| Related structure data |  9gkmMC  9gknC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_51429.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_51429.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | deepEMhancer-sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8512 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_51429_msk_1.map emd_51429_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map from non-uniform refinement
| File | emd_51429_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map from non-uniform refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 from non-uniform refinement
| File | emd_51429_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 from non-uniform refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 from non-uniform refinement
| File | emd_51429_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 from non-uniform refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TRIP12 deltaN K29/K48-branched chain formation complex
| Entire | Name: TRIP12 deltaN K29/K48-branched chain formation complex |
|---|---|
| Components |
|
-Supramolecule #1: TRIP12 deltaN K29/K48-branched chain formation complex
| Supramolecule | Name: TRIP12 deltaN K29/K48-branched chain formation complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 200 KDa |
-Macromolecule #1: Ubiquitin
| Macromolecule | Name: Ubiquitin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 8.519778 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQIFVKTLTG KTITLEVEPS DTIENVKAKI QDKEGIPPDQ QRLIFAGKQL EDGRTLSDYN IQKESTLHLV LRLRG UniProtKB: Polyubiquitin-C |
-Macromolecule #2: Polyubiquitin-C
| Macromolecule | Name: Polyubiquitin-C / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 8.550794 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQIFVKTLTG KTITLEVEPS DTIENVKACI QDKEGIPPDQ QRLIFAGKQL EDGRTLSDYN IQKESTLHLV LRLRGG UniProtKB: Polyubiquitin-C |
-Macromolecule #3: Polyubiquitin-B
| Macromolecule | Name: Polyubiquitin-B / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 8.604845 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQIFVKTLTG KTITLEVEPS DTIENVKAKI QDKEGIPPDQ QRLIFAGRQL EDGRTLSDYN IQKESTLHLV LRLRGG UniProtKB: Polyubiquitin-B |
-Macromolecule #4: Isoform 3 of E3 ubiquitin-protein ligase TRIP12
| Macromolecule | Name: Isoform 3 of E3 ubiquitin-protein ligase TRIP12 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO / EC number: HECT-type E3 ubiquitin transferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 175.752453 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSTIGSGASS KAQQLLQGLQ ASDESQQLQA VIEMCQLLVM GNEETLGGFP VKSVVPALIT LLQMEHNFDI MNHACRALTY MMEALPRSS AVVVDAIPVF LEKLQVIQCI DVAEQALTAL EMLSRRHSKA ILQAGGLADC LLYLEFFSIN AQRNALAIAA N CCQSITPD ...String: GSTIGSGASS KAQQLLQGLQ ASDESQQLQA VIEMCQLLVM GNEETLGGFP VKSVVPALIT LLQMEHNFDI MNHACRALTY MMEALPRSS AVVVDAIPVF LEKLQVIQCI DVAEQALTAL EMLSRRHSKA ILQAGGLADC LLYLEFFSIN AQRNALAIAA N CCQSITPD EFHFVADSLP LLTQRLTHQD KKSVESTCLC FARLVDNFQH EENLLQQVAS KDLLTNVQQL LVVTPPILSS GM FIMVVRM FSLMCSNCPT LAVQLMKQNI AETLHFLLCG ASNGSCQEQI DLVPRSPQEL YELTSLICEL MPCLPKEGIF AVD TMLKKG NAQNTDGAIW QWRDDRGLWH PYNRIDSRII EQINEDTGTA RAIQRKPNPL ANSNTSGYSE SKKDDARAQL MKED PELAK SFIKTLFGVL YEVYSSSAGP AVRHKCLRAI LRIIYFADAE LLKDVLKNHA VSSHIASMLS SQDLKIVVGA LQMAE ILMQ KLPDIFSVYF RREGVMHQVK HLAESESLLT SPPKACTNGS GSMGSTTSVS SGTATAATHA AADLGSPSLQ HSRDDS LDL SPQGRLSDVL KRKRLPKRGP RRPKYSPPRD DDKVDNQAKS PTTTQSPKSS FLASLNPKTW GRLSTQSNSN NIEPART AG GSGLARAASK DTISNNREKI KGWIKEQAHK FVERYFSSEN MDGSNPALNV LQRLCAATEQ LNLQVDGGAE CLVEIRSI V SESDVSSFEI QHSGFVKQLL LYLTSKSEKD AVSREIRLKR FLHVFFSSPL PGEEPIGRVE PVGNAPLLAL VHKMNNCLS QMEQFPVKVH DFPSGNGTGG SFSLNRGSQA LKFFNTHQLK CQLQRHPDCA NVKQWKGGPV KIDPLALVQA IERYLVVRGY GRVREDDED SDDDGSDEEI DESLAAQFLN SGNVRHRLQF YIGEHLLPYN MTVYQAVRQF SIQAEDERES TDDESNPLGR A GIWTKTHT IWYKPVREDE ESNKDCVGGK RGRAQTAPTK TSPRNAKKHD ELWHDGVCPS VSNPLEVYLI PTPPENITFE DP SLDVILL LRVLHAISRY WYYLYDNAMC KEIIPTSEFI NSKLTAKANR QLQDPLVIMT GNIPTWLTEL GKTCPFFFPF DTR QMLFYV TAFDRDRAMQ RLLDTNPEIN QSDSQDSRVA PRLDRKKRTV NREELLKQAE SVMQDLGSSR AMLEIQYENE VGTG LGPTL EFYALVSQEL QRADLGLWRG EEVTLSNPKG SQEGTKYIQN LQGLFALPFG RTAKPAHIAK VKMKFRFLGK LMAKA IMDF RLVDLPLGLP FYKWMLRQET SLTSHDLFDI DPVVARSVYH LEDIVRQKKR LEQDKSQTKE SLQYALETLT MNGCSV EDL GLDFTLPGFP NIELKKGGKD IPVTIHNLEE YLRLVIFWAL NEGVSRQFDS FRDGFESVFP LSHLQYFYPE ELDQLLC GS KADTWDAKTL MECCRPDHGY THDSRAVKFL FEILSSFDNE QQRLFLQFVT GSPRLPVGGF RSLNPPLTIV RKTFESTE N PDDFLPSVMT CVNYLKLPDY SSIEIMREKL LIAAREGQQS FHLS UniProtKB: E3 ubiquitin-protein ligase TRIP12 |
-Macromolecule #5: 5-azanylpentan-2-one
| Macromolecule | Name: 5-azanylpentan-2-one / type: ligand / ID: 5 / Number of copies: 1 / Formula: SY8 |
|---|---|
| Molecular weight | Theoretical: 101.147 Da |
| Chemical component information | 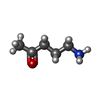 ChemComp-SY8: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 66.84 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















































