[English] 日本語
 Yorodumi
Yorodumi- EMDB-50938: Lysosomal transporting complex of beta-glucocerebrosidase (GCase)... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Lysosomal transporting complex of beta-glucocerebrosidase (GCase) and lysosomal integral membrane protein 2 (LIMP-2) with bound Pro-macrobodies (LIMP-2 local refinement) | ||||||||||||||||||
 Map data Map data | Focused refinement of LIMP-2 in complex with GCase and two bound Pro-macrobodies. Sharpened map (b-factor:-130.5) obtained from local refinement. | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Parkinson / lysosome / Gaucher / GCase / SCARB2 / Pro-macrobody / Glucosylceramide / complex / TRANSPORT PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of glucosylceramide catabolic process / regulation of carbohydrate catabolic process / regulation of endosome organization / steryl-beta-glucosidase activity / positive regulation of neuronal action potential / beta-glucoside catabolic process / cerebellar Purkinje cell layer formation / termination of signal transduction / galactosylceramidase / galactosylceramidase activity ...regulation of glucosylceramide catabolic process / regulation of carbohydrate catabolic process / regulation of endosome organization / steryl-beta-glucosidase activity / positive regulation of neuronal action potential / beta-glucoside catabolic process / cerebellar Purkinje cell layer formation / termination of signal transduction / galactosylceramidase / galactosylceramidase activity / aminophospholipid transport / : / glucosylceramidase / lymphocyte migration / scavenger receptor binding / glucosylceramide catabolic process / regulation of lysosomal protein catabolic process / sphingosine biosynthetic process / response to thyroid hormone / autophagosome organization / glucosylceramidase activity / microglial cell proliferation / lysosomal protein catabolic process / glucosyltransferase activity / endosome to plasma membrane protein transport / regulation of TOR signaling / regulation of lysosome organization / Glycosphingolipid catabolism / protein targeting to lysosome / microglia differentiation / lipid storage / phosphatidylcholine binding / positive regulation of type 2 mitophagy / ceramide biosynthetic process / brain morphogenesis / Hydrolases; Glycosylases; Glycosidases, i.e. enzymes that hydrolyse O- and S-glycosyl compounds / response to pH / pyramidal neuron differentiation / cargo receptor activity / negative regulation of protein metabolic process / scavenger receptor activity / Transferases; Glycosyltransferases; Hexosyltransferases / lysosome organization / cholesterol binding / phosphatidylserine binding / response to dexamethasone / neuromuscular process / antigen processing and presentation / hematopoietic stem cell proliferation / response to testosterone / Association of TriC/CCT with target proteins during biosynthesis / motor behavior / negative regulation of interleukin-6 production / establishment of skin barrier / homeostasis of number of cells / regulation of macroautophagy / negative regulation of protein-containing complex assembly / cholesterol metabolic process / mitophagy / negative regulation of MAPK cascade / cell maturation / receptor-mediated endocytosis / lysosomal lumen / cellular response to starvation / determination of adult lifespan / respiratory electron transport chain / trans-Golgi network / clathrin-coated endocytic vesicle membrane / sensory perception of sound / positive regulation of neuron projection development / autophagy / response to estrogen / negative regulation of inflammatory response / cellular response to tumor necrosis factor / endocytic vesicle membrane / transmembrane signaling receptor activity / T cell differentiation in thymus / Cargo recognition for clathrin-mediated endocytosis / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / late endosome membrane / protein-folding chaperone binding / Clathrin-mediated endocytosis / virus receptor activity / neuron apoptotic process / negative regulation of neuron apoptotic process / proteasome-mediated ubiquitin-dependent protein catabolic process / lysosome / endosome membrane / Golgi membrane / signaling receptor binding / lysosomal membrane / focal adhesion / endoplasmic reticulum membrane / enzyme binding / endoplasmic reticulum / Golgi apparatus / protein homodimerization activity / extracellular exosome / membrane / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / unidentified (others) Homo sapiens (human) / unidentified (others) | ||||||||||||||||||
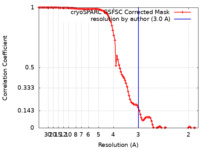
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | ||||||||||||||||||
 Authors Authors | Dobert JP / Schaefer JHS / Dal Maso T / Socher E / Versees W / Moeller A / Zunke F / Arnold P | ||||||||||||||||||
| Funding support |  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Cryo-TEM structure of β-glucocerebrosidase in complex with its transporter LIMP-2. Authors: Jan Philipp Dobert / Jan-Hannes Schäfer / Thomas Dal Maso / Priyadarshini Ravindran / Dustin J E Huard / Eileen Socher / Lisa A Schildmeyer / Raquel L Lieberman / Wim Versées / Arne ...Authors: Jan Philipp Dobert / Jan-Hannes Schäfer / Thomas Dal Maso / Priyadarshini Ravindran / Dustin J E Huard / Eileen Socher / Lisa A Schildmeyer / Raquel L Lieberman / Wim Versées / Arne Moeller / Friederike Zunke / Philipp Arnold /    Abstract: Targeting proteins to their final cellular destination requires transport mechanisms and nearly all lysosomal enzymes reach the lysosome via the mannose-6-phosphate receptor pathway. One of the few ...Targeting proteins to their final cellular destination requires transport mechanisms and nearly all lysosomal enzymes reach the lysosome via the mannose-6-phosphate receptor pathway. One of the few known exceptions is the enzyme β-glucocerebrosidase (GCase) that requires the lysosomal integral membrane protein type-2 (LIMP-2) as a proprietary lysosomal transporter. Genetic variations in the GCase encoding gene GBA1 cause Gaucher's disease (GD) and present the highest genetic risk factor to develop Parkinson's disease (PD). Activators targeting GCase emerge as a promising therapeutic approach to treat GD and PD, with pre-clinical and clinical trials ongoing. In this study, we resolve the complex of GCase and LIMP-2 using cryo-electron microscopy with the aid of an engineered LIMP-2 shuttle and two GCase-targeted pro-macrobodies. We identify helix 5 and helix 7 of LIMP-2 to interact with a binding pocket in GCase, forming a mostly hydrophobic interaction interface supported by one essential salt bridge. Understanding the interplay of GCase and LIMP-2 on a structural level is crucial to identify potential activation sites and conceptualizing novel therapeutic approaches targeting GCase. Here, we unveil the protein structure of a mannose-6-phosphate-independent lysosomal transport complex and provide fundamental knowledge for translational clinical research to overcome GD and PD. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50938.map.gz emd_50938.map.gz | 117.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50938-v30.xml emd-50938-v30.xml emd-50938.xml emd-50938.xml | 23.7 KB 23.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50938_fsc.xml emd_50938_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_50938.png emd_50938.png | 123.6 KB | ||
| Filedesc metadata |  emd-50938.cif.gz emd-50938.cif.gz | 7.1 KB | ||
| Others |  emd_50938_half_map_1.map.gz emd_50938_half_map_1.map.gz emd_50938_half_map_2.map.gz emd_50938_half_map_2.map.gz | 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50938 http://ftp.pdbj.org/pub/emdb/structures/EMD-50938 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50938 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50938 | HTTPS FTP |
-Related structure data
| Related structure data |  9fjfC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_50938.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50938.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused refinement of LIMP-2 in complex with GCase and two bound Pro-macrobodies. Sharpened map (b-factor:-130.5) obtained from local refinement. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.925 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map B
| File | emd_50938_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_50938_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Lysosomal transporting complex of beta-glucocerebrosidase and lys...
| Entire | Name: Lysosomal transporting complex of beta-glucocerebrosidase and lysosomal integral membrane protein 2 (LIMP-2) with two bound Pro-macrobodies. |
|---|---|
| Components |
|
-Supramolecule #1: Lysosomal transporting complex of beta-glucocerebrosidase and lys...
| Supramolecule | Name: Lysosomal transporting complex of beta-glucocerebrosidase and lysosomal integral membrane protein 2 (LIMP-2) with two bound Pro-macrobodies. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Maltose-binding protein (MBP) domains of Pro-macrobodies were not resolved, only nanobody domains are included in the model. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 250 KDa |
-Macromolecule #1: Lysosomal acid glucosylceramidase
| Macromolecule | Name: Lysosomal acid glucosylceramidase / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: glucosylceramidase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ARPCIPKSFG YSSVVCVCNA TYCDSFDPPT FPALGTFSRY ESTRSGRRME LSMGPIQANH TGTGLLLTLQ PEQKFQKVKG FGGAMTDAA ALNILALSPP AQNLLLKSYF SEEGIGYNII RVPMASCDFS IRTYTYADTP DDFQLHNFSL PEEDTKLKIP L IHRALQLA ...String: ARPCIPKSFG YSSVVCVCNA TYCDSFDPPT FPALGTFSRY ESTRSGRRME LSMGPIQANH TGTGLLLTLQ PEQKFQKVKG FGGAMTDAA ALNILALSPP AQNLLLKSYF SEEGIGYNII RVPMASCDFS IRTYTYADTP DDFQLHNFSL PEEDTKLKIP L IHRALQLA QRPVSLLASP WTSPTWLKTN GAVNGKGSLK GQPGDIYHQT WARYFVKFLD AYAEHKLQFW AVTAENEPSA GL LSGYPFQ CLGFTPEHQR DFIARDLGPT LANSTHHNVR LLMLDDQRLL LPHWAKVVLT DPEAAKYVHG IAVHWYLDFL APA KATLGE THRLFPNTML FASEACVGSK FWEQSVRLGS WDRGMQYSHS IITNLLYHVV GWTDWNLALN PEGGPNWVRN FVDS PIIVD ITKDTFYKQP MFYHLGHFSK FIPEGSQRVG LVASQKNDLD AVALMHPDGS AVVVVLNRSS KDVPLTIKDP AVGFL ETIS PGYSIHTYLW RRQ UniProtKB: Lysosomal acid glucosylceramidase |
-Macromolecule #2: Nanobody Nb6
| Macromolecule | Name: Nanobody Nb6 / type: protein_or_peptide / ID: 2 Details: Pro-Macrobody; nanobody fused to maltose-binding protein (MBP) via Pro-Pro linker; MBP not resolved and not modeled. Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: unidentified (others) |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLVESGGG LVQPGGSLRL SCAASGSIFS INTMGWYRQA PGKEREMVAY IITFGSTNYA DSVKGRFTIS GDNANNTMWL QMNSLKPED TAVYYCYAAI RPTDSSTYTS YWGQGTQVTV PP |
-Macromolecule #3: Lysosomal membrane protein 2
| Macromolecule | Name: Lysosomal membrane protein 2 / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: KKIVLRNGTE AFDSWEKPPL PVYTQFYFFN VTNPEEILRG ETPRVEEVGP YTYRELRNKA NIQFGDNGTT ISAVSNKAYV FERDQSVGD PKIDLIRTLN IPVLTVIEWS QVHFLREIIE AMLKAYQQKL FVTHTVDELL WGYKDEILSL IHVFRPDISP Y FGLFYEKN ...String: KKIVLRNGTE AFDSWEKPPL PVYTQFYFFN VTNPEEILRG ETPRVEEVGP YTYRELRNKA NIQFGDNGTT ISAVSNKAYV FERDQSVGD PKIDLIRTLN IPVLTVIEWS QVHFLREIIE AMLKAYQQKL FVTHTVDELL WGYKDEILSL IHVFRPDISP Y FGLFYEKN GTNDGDYVFL TGEDSYLNFT KIVEWNGKTS LDWWITDKCN MINGTDGDSF HPLITKDEVL YVFPSDFCRS VY ITFSDYE SVQGLPAFRY KVPAEILANT SDNAGFCIPE GNCLGSGVLN VSICKNGAPI IMSFPHFYQA DERFVSYLDF LAP NQEDHE TFVDINPLTG IILKAAKRFQ INIYVKKLDD FVETGDIRTM VFPVMYLNES VHIDKETASR LKSMI UniProtKB: Lysosome membrane protein 2 |
-Macromolecule #4: Nanobody Nb1
| Macromolecule | Name: Nanobody Nb1 / type: protein_or_peptide / ID: 4 Details: Pro-Macrobody; nanobody fused to maltose-binding protein (MBP) via Pro-Pro linker; MBP not resolved and not modeled. Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: unidentified (others) |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLVESGGG LVQPGGSLRL SCAASGFTLD YYAIGWFRQA PGKEREGVSC ISSSDGSTYY ADSAKGRFTI SRDNAKNTVY LQMNSLKPE DTAVYYCATD RGQCTYYSSG YYRDLRWYDY WGQGTQVTVP P |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.6 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: 20 mM MES; 150 mM NaCl, pH: 7.4 | |||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 2 | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||
| Details | Monodisperse, complex seperated from monomers via size exclusion chhromatography. |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 10550 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 130000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: AAA / Chain - Residue range: 1-497 / Chain - Source name: PDB / Chain - Initial model type: experimental model / Details: GCase |
|---|---|
| Details | Initial fitting with ChimeraX, manual flexible fitting with Coot, refinement with Phenix |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
-Atomic model buiding 2
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 37-430 / Chain - Source name: PDB / Chain - Initial model type: experimental model / Details: LIMP-2 |
|---|---|
| Details | Initial fitting with ChimeraX, manual flexible fitting with Coot, refinement with Phenix |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
-Atomic model buiding 3
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model / Details: Nanobody Nb1; Unpublished |
|---|---|
| Details | Initial fitting with ChimeraX, manual flexible fitting with Coot, refinement with Phenix |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)