+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The structure of GluA1/A4 LBD-TMD with 2 TARPs | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | iGluR / CP-AMPA receptors / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationCargo concentration in the ER / Presynaptic depolarization and calcium channel opening / axonal spine / positive regulation of locomotion involved in locomotory behavior / positive regulation of membrane potential / COPII-mediated vesicle transport / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cellular response to ammonium ion / response to sucrose ...Cargo concentration in the ER / Presynaptic depolarization and calcium channel opening / axonal spine / positive regulation of locomotion involved in locomotory behavior / positive regulation of membrane potential / COPII-mediated vesicle transport / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cellular response to ammonium ion / response to sucrose / neuron spine / myosin V binding / cerebellar mossy fiber / postsynaptic neurotransmitter receptor diffusion trapping / proximal dendrite / channel regulator activity / LGI-ADAM interactions / regulation of monoatomic ion transmembrane transport / Trafficking of AMPA receptors / regulation of AMPA receptor activity / response to arsenic-containing substance / kainate selective glutamate receptor complex / cellular response to L-glutamate / cellular response to dsRNA / membrane hyperpolarization / dendritic spine membrane / regulation of synapse structure or activity / beta-2 adrenergic receptor binding / nervous system process / long-term synaptic depression / Synaptic adhesion-like molecules / protein targeting to membrane / cellular response to peptide hormone stimulus / voltage-gated calcium channel complex / neuronal cell body membrane / cellular response to amine stimulus / response to psychosocial stress / peptide hormone receptor binding / response to morphine / neurotransmitter receptor localization to postsynaptic specialization membrane / spinal cord development / Activation of AMPA receptors / perisynaptic space / neuromuscular junction development / protein kinase A binding / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors / response to lithium ion / negative regulation of smooth muscle cell apoptotic process / behavioral response to pain / transmission of nerve impulse / adenylate cyclase binding / immunoglobulin binding / asymmetric synapse / AMPA glutamate receptor complex / response to electrical stimulus / regulation of receptor recycling / ionotropic glutamate receptor complex / membrane depolarization / conditioned place preference / G-protein alpha-subunit binding / Unblocking of NMDA receptors, glutamate binding and activation / glutamate receptor binding / long-term memory / positive regulation of synaptic transmission / regulation of postsynaptic membrane neurotransmitter receptor levels / positive regulation of synaptic transmission, glutamatergic / postsynaptic density, intracellular component / response to fungicide / neuronal action potential / voltage-gated calcium channel activity / glutamate-gated receptor activity / cellular response to brain-derived neurotrophic factor stimulus / synapse assembly / somatodendritic compartment / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / dendrite membrane / ionotropic glutamate receptor binding / excitatory synapse / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / synaptic membrane / positive regulation of excitatory postsynaptic potential / dendritic shaft / hippocampal mossy fiber to CA3 synapse / response to cocaine / PDZ domain binding / calcium channel regulator activity / neuromuscular junction / regulation of membrane potential / synaptic transmission, glutamatergic / cellular response to amino acid stimulus / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / response to nutrient levels / response to calcium ion / recycling endosome / regulation of synaptic plasticity / cerebral cortex development / receptor internalization / postsynaptic density membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.22 Å | |||||||||
 Authors Authors | Fang CL / Gouaux E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2025 Journal: Nature / Year: 2025Title: Gating and noelin clustering of native Ca-permeable AMPA receptors. Authors: Chengli Fang / Cathy J Spangler / Jumi Park / Natalie Sheldon / Laurence O Trussell / Eric Gouaux /  Abstract: AMPA-type ionotropic glutamate receptors (AMPARs) are integral to fast excitatory synaptic transmission and have vital roles in synaptic plasticity, motor coordination, learning and memory. Whereas ...AMPA-type ionotropic glutamate receptors (AMPARs) are integral to fast excitatory synaptic transmission and have vital roles in synaptic plasticity, motor coordination, learning and memory. Whereas extensive structural studies have been conducted on recombinant AMPARs and native calcium-impermeable (CI)-AMPARs alongside their auxiliary proteins, the molecular architecture of native calcium-permeable (CP)-AMPARs has remained undefined. Here, to determine the subunit composition, physiological architecture and gating mechanisms of CP-AMPARs, we visualize these receptors, immunoaffinity purified from rat cerebella, and resolve their structures using cryo-electron microscopy (cryo-EM). Our results indicate that the predominant assembly consists of GluA1 and GluA4 subunits, with the GluA4 subunit occupying the B and D positions, and auxiliary subunits, including transmembrane AMPAR regulatory proteins (TARPs) located at the B' and D' positions, and cornichon homologues (CNIHs) or TARPs located at the A' and C' positions. Furthermore, we resolved the structure of the noelin (NOE1)-GluA1-GluA4 complex, in which NOE1 specifically binds to the GluA4 subunit at the B and D positions. Notably, NOE1 stabilizes the amino-terminal domain layer without affecting gating properties of the receptor. NOE1 contributes to AMPAR function by forming dimeric AMPAR assemblies that are likely to engage in extracellular networks, clustering receptors in synaptic environments and modulating receptor responsiveness to synaptic inputs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_49726.map.gz emd_49726.map.gz | 40.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-49726-v30.xml emd-49726-v30.xml emd-49726.xml emd-49726.xml | 22.6 KB 22.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_49726_fsc.xml emd_49726_fsc.xml | 7.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_49726.png emd_49726.png | 93.9 KB | ||
| Filedesc metadata |  emd-49726.cif.gz emd-49726.cif.gz | 6.6 KB | ||
| Others |  emd_49726_additional_1.map.gz emd_49726_additional_1.map.gz emd_49726_half_map_1.map.gz emd_49726_half_map_1.map.gz emd_49726_half_map_2.map.gz emd_49726_half_map_2.map.gz | 21.7 MB 39.6 MB 39.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-49726 http://ftp.pdbj.org/pub/emdb/structures/EMD-49726 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-49726 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-49726 | HTTPS FTP |
-Related structure data
| Related structure data |  9nr9MC  9nr6C  9nr7C  9nr8C  9nraC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_49726.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_49726.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.88 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_49726_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_49726_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_49726_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CP-AMPA receptors
| Entire | Name: CP-AMPA receptors |
|---|---|
| Components |
|
-Supramolecule #1: CP-AMPA receptors
| Supramolecule | Name: CP-AMPA receptors / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Voltage-dependent calcium channel gamma-2 subunit
| Macromolecule | Name: Voltage-dependent calcium channel gamma-2 subunit / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.589975 KDa |
| Sequence | String: DRGVQMLLTT VGAFAAFSLM TIAVGTDYWL YSRGVCKTKS VSENETSKKN EEVMTHSGLW RTCCLEGNFK GLCKQIDHFP EDADYEADT AEYFLRAVRA SSIFPILSVI LLFMGGLCIA ASEFYKTRHN IILSAGIFFV SAGLSNIIGI IVYISANAGD P SKSDSKKN ...String: DRGVQMLLTT VGAFAAFSLM TIAVGTDYWL YSRGVCKTKS VSENETSKKN EEVMTHSGLW RTCCLEGNFK GLCKQIDHFP EDADYEADT AEYFLRAVRA SSIFPILSVI LLFMGGLCIA ASEFYKTRHN IILSAGIFFV SAGLSNIIGI IVYISANAGD P SKSDSKKN SYSYGWSFYF GALSFIIAEM VGVLAVHMFI DRHKQL UniProtKB: Voltage-dependent calcium channel gamma-2 subunit |
-Macromolecule #2: Glutamate receptor 1
| Macromolecule | Name: Glutamate receptor 1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47.879855 KDa |
| Sequence | String: RTYIVTTILE DPYVMLKKNA NQFEGNDRYE GYCVELAAEI AKHVGYSYRL EIVSDGKYGA RDPDTKAWNG MVGELVYGRA DVAVAPLTI TLVREEVIDF SKPFMSLGIS IMIKKPQKSK PGVFSFLDPL AYEIWMCIVF AYIGVSVVLF LVSRFSPYEW H SEEFEEGR ...String: RTYIVTTILE DPYVMLKKNA NQFEGNDRYE GYCVELAAEI AKHVGYSYRL EIVSDGKYGA RDPDTKAWNG MVGELVYGRA DVAVAPLTI TLVREEVIDF SKPFMSLGIS IMIKKPQKSK PGVFSFLDPL AYEIWMCIVF AYIGVSVVLF LVSRFSPYEW H SEEFEEGR DQTTSDQSNE FGIFNSLWFS LGAFMQQGCD ISPRSLSGRI VGGVWWFFTL IIISSYTANL AAFLTVERMV SP IESAEDL AKQTEIAYGT LEAGSTKEFF RRSKIAVFEK MWTYMKSAEP SVFVRTTEEG MIRVRKSKGK YAYLLESTMN EYI EQRKPC DTMKVGGNLD SKGYGIATPK GSALRNPVNL AVLKLNEQGL LDKLKNKWWY DKGECGSGGG DSKDKTSALS LSNV AGVFY ILIGGLGLAM LVALIEFCYK SR UniProtKB: Glutamate receptor 1 |
-Macromolecule #3: Isoform 2 of Glutamate receptor 4
| Macromolecule | Name: Isoform 2 of Glutamate receptor 4 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47.173348 KDa |
| Sequence | String: VVVTTIMESP YVMYKKNHEM FEGNDKYEGY CVDLASEIAK HIGIKYKIAI VPDGKYGARD ADTKIWNGMV GELVYGKAEI AIAPLTITL VREEVIDFSK PFMSLGISIM IKKPQKSKPG VFSFLDPLAY EIWMCIVFAY IGVSVVLFLV SRFSPYEWHT E EPEDGKEG ...String: VVVTTIMESP YVMYKKNHEM FEGNDKYEGY CVDLASEIAK HIGIKYKIAI VPDGKYGARD ADTKIWNGMV GELVYGKAEI AIAPLTITL VREEVIDFSK PFMSLGISIM IKKPQKSKPG VFSFLDPLAY EIWMCIVFAY IGVSVVLFLV SRFSPYEWHT E EPEDGKEG PSDQPPNEFG IFNSLWFSLG AFMQQGCDIS PRSLSGRIVG GVWWFFTLII ISSYTANLAA FLTVERMVSP IE SAEDLAK QTEIAYGTLD SGSTKEFFRR SKIAVYEKMW TYMRSAEPSV FTRTTAEGVA RVRKSKGKFA FLLESTMNEY TEQ RKPCDT MKVGGNLDSK GYGVATPKGS SLRTPVNLAV LKLSEAGVLD KLKNKWWYDK GECGPKDSGS KDKTSALSLS NVAG VFYIL VGGLGLAMLV ALIEFCYKS UniProtKB: Glutamate receptor 4 |
-Macromolecule #4: {[7-morpholin-4-yl-2,3-dioxo-6-(trifluoromethyl)-3,4-dihydroquino...
| Macromolecule | Name: {[7-morpholin-4-yl-2,3-dioxo-6-(trifluoromethyl)-3,4-dihydroquinoxalin-1(2H)-yl]methyl}phosphonic acid type: ligand / ID: 4 / Number of copies: 4 / Formula: ZK1 |
|---|---|
| Molecular weight | Theoretical: 409.254 Da |
| Chemical component information | 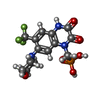 ChemComp-ZK1: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: OTHER / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)













































